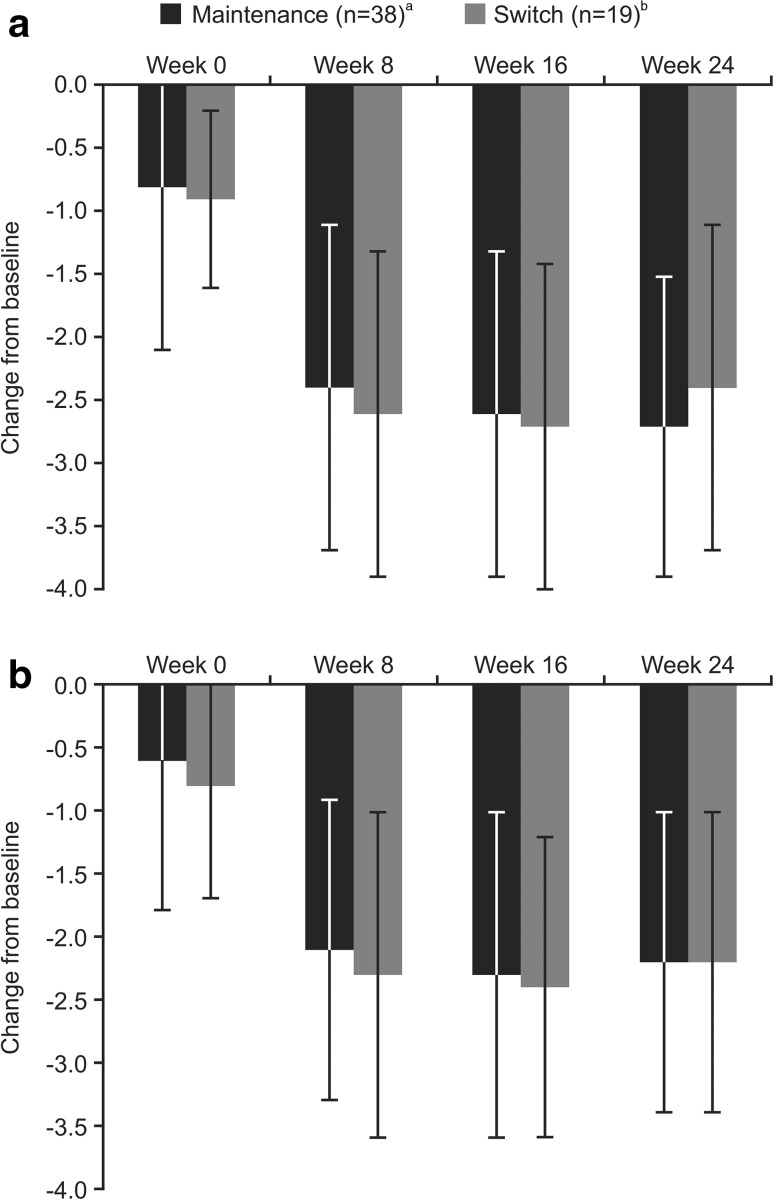
Fig. 2.
Mean (standard deviation) change in a DAS28-ESR and b DAS28-CRP from baseline in patients who received CT-P10 during the OLE study (efficacy population). Baseline refers to week 0 of the preceding phase I RCT. aPatients treated with CT-P10 during the preceding phase I RCT and also during the OLE study. bPatients treated with RTX during the preceding phase I RCT and with CT-P10 during the OLE study. One patient was excluded from the efficacy population due to major protocol deviations [non-compliance with the eligibility criteria (received >2 biologic agents prior to start of study) plus received prohibited medication after study commencement]. CRP C-reactive protein, DAS28 Disease Activity Score using 28 joints, ESR erythrocyte sedimentation rate, OLE open-label extension, RCT randomized controlled trial, RTX innovator rituximab