Figure 1.
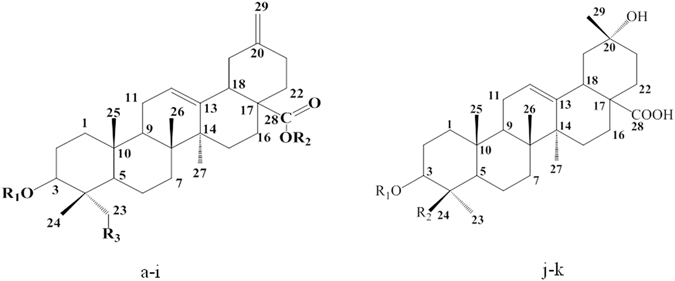
The structures of compounds isolated from the EtOH extracts of S. brachyanthera. (a) brachyantheraoside A1 R1 = ara- R2 = glc1-6glc- R3 = OH. (b) brachyantheraoside A3 R1 = glcaOMe1-3glc- R2 = rha1-4glc1-6glc- R3 = OH. (c) brachyantheraoside A4 R1 = ara1-4ara1-3ara- R2 = rha1-4glc1-6glc- R3 = H. (d) brachyantheraoside A5 R1 = ara1-(xyl1-2) 3ara1-3ara- R2 = rha1-4glc1-6glc- R3 = H. (e) YM7 R1 = glc1-3rha1-2ara- R2 = rha1-4glc1-6glc- R3 = H. (f) YM9 R1 = rha1-2ara- R2 = rha1-4glc1-6glc- R3 = H. (g) YM10 R1 = ara- R2 = glc1-6glc- R3 = H. (h) YM11 R1 = rha1-2ara- R2 = glc1-6glc- R3 = H. (i) YM13 R1 = ara- R2 = rha1-4glc1-6glc- R3 = H. (j) brachyantheraoside B6 R1 = H R2 = rha1-2glc-O-. (k) brachyantheraoside B9 R1 = ara1-(rha1-2) 3glc- R2 = H.
