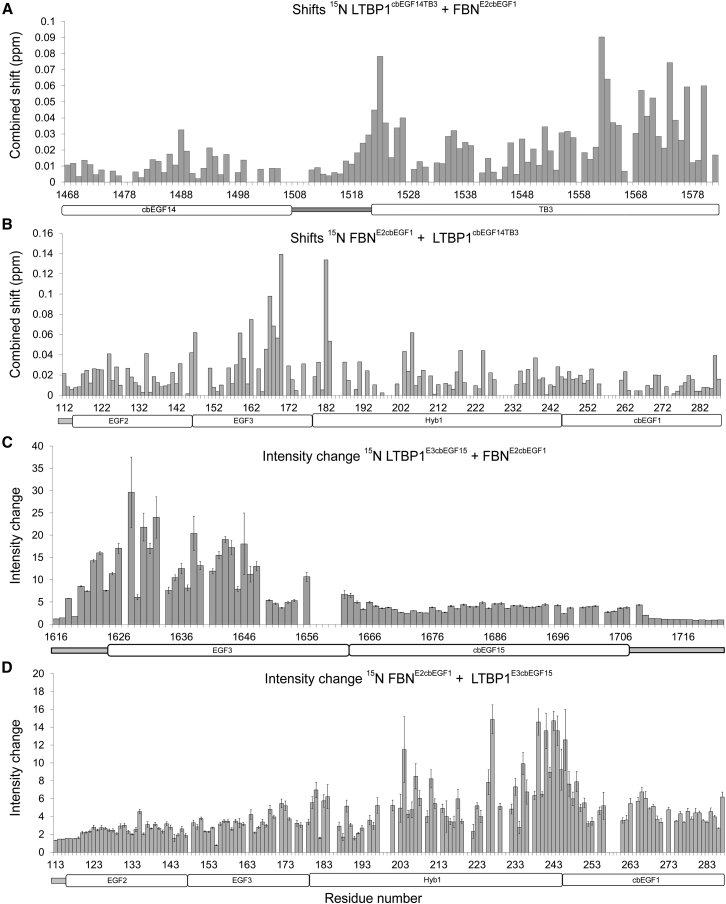
Figure 3.
HSQC Titration Data Highlights Multiple Binding Sites in Both FBN1 and LTBP1
Titrations were carried out by sequential addition of lyophilized unlabeled protein to 15N-labeled protein samples and monitored using 1H-15N HSQC spectra.
(A and B) Peaks were observed to shift in titrations of (A) 15N-LTBP1cbEGF14TB3 with FBN1E2cbEGF1 added, or of (B) 15N-FBN1E2cbEGF1 with LTBP1cbEGF14TB3 added. The combined 1HN and 15N chemical shift change is plotted as a function of protein sequence.
(C and D) Peak broadening was observed in titrations of (C) 15N-LTBP1E3cbEGF15 with FBN1E2cbEGF1 added or of (D) 15N-FBN1E2cbEGF1 with LTBP1E3cbEGF15 added. Peak intensity changes, measured as the ratio of peak intensity in the absence of ligand to that in the presence of ligand, are plotted as a function of protein sequence. Error bars are determined from the effect of background noise on peak height (SD). Gaps in the plots occur for residues with unassigned or very weak peaks in the HSQC or for prolines.