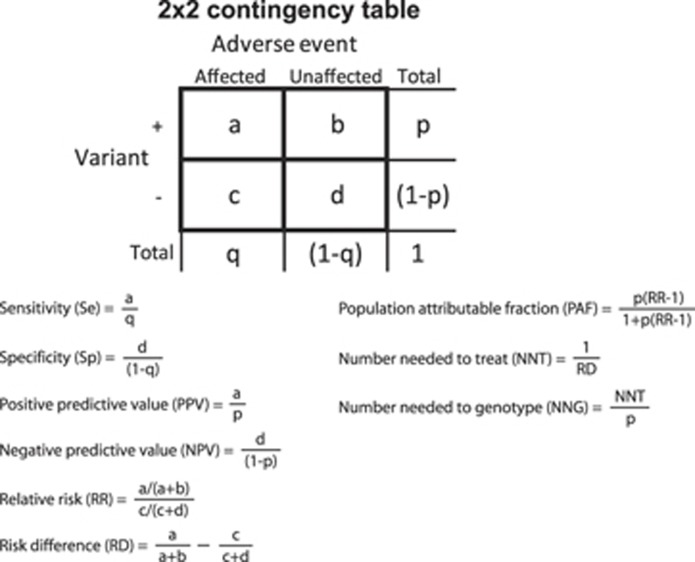
Figure 1.
Calculation of clinical validity and potential population impact measures from 2 × 2 contingency tables reporting adverse event by genetic variant subgroups. Contingency tables can be constructed using empirical data or using hypothetical data calculated from summary statistics and association measures, such as odds ratios derived from observational studies with a case–control design in combination with the frequencies of the genetic variant and the adverse event (see Supplementary Information).