Figure 3. Phe-bound PAH ACT domain dimer.
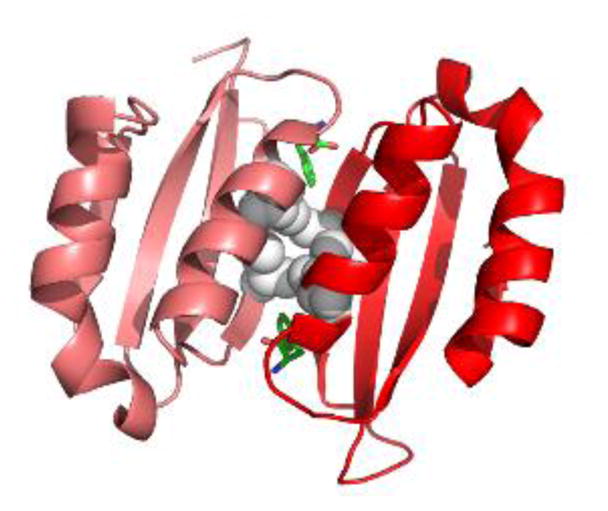
The crystal structure of a truncated human PAH ACT domain (PDB id 5FII, containing residues 34–111 (19)) forms a dimer and shows Phe (green) binding as predicted (18). The α-helices are predicted to be solvent exposed while the 8-stranded β-sheet is predicted to face into the tetramer. Grey balls are used for the side chains of Leu48 and Ile65. These four interacting residues contribute to the hydrophobic core of the ACT-domain dimer. L48S and I65T are each predicted to disrupt this important stabilizing interaction. Leu48 and Ile65 each have van der Waals interactions with the allosteric Phe.
