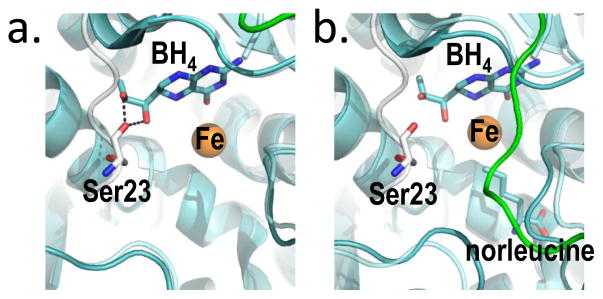
Figure 5. BH4, bound two different ways at the PAH active site provides the molecular basis for stabilization of RS-PAH.
(a) An overlay of crystal structures (full length RS-PAH (white); PDB id 5DEN and PAH catalytic domain containing BH4 (cyan/green); PDB id 1J8U) illustrates that BH4 is positioned to make two stabilizing H-bonds to Ser23 which secure the auto-inhibitory interaction. (b) An alternate overlay (full length PAH (white); PDB id 5DEN and PAH catalytic domain containing BH4 and norleucine (cyan/green); PDB id 1MMT) shows BH4 sitting deeper in the active site and too far away to make the stabilizing H-bonds.