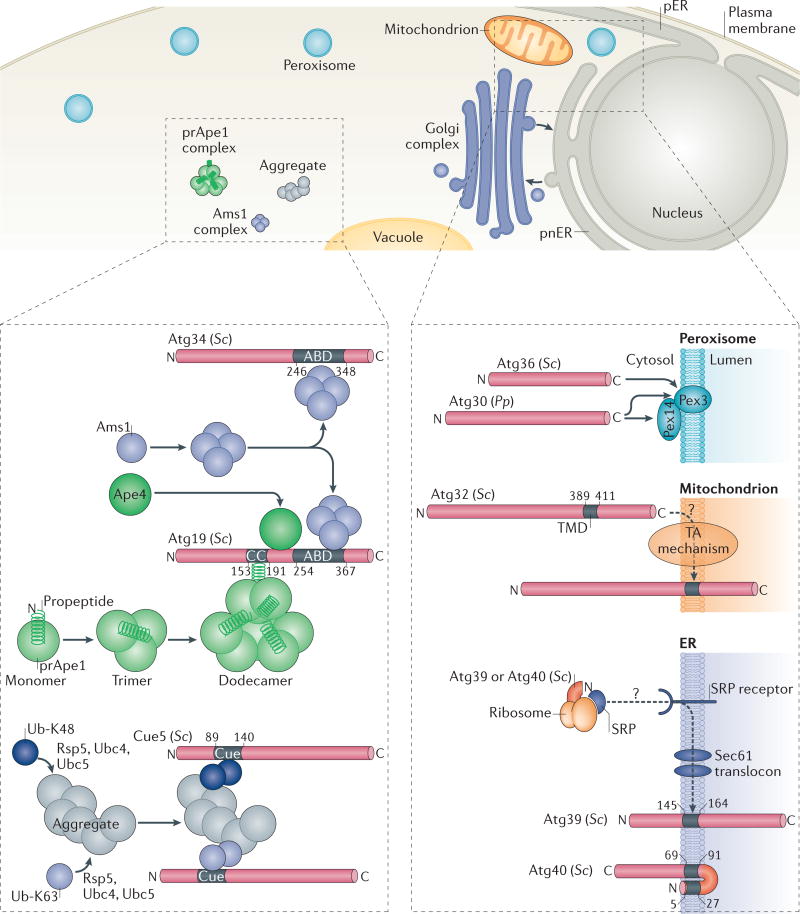
Figure 2. Selective autophagy pathways and cargo recognition by selective autophagy receptors.
The upper panel of the figure shows the specific cargoes, such as oligomeric α-mannosidase 1 (Ams1) or precursor of vacuolar aminopeptidase 1 (prApe1), protein aggregates or organelles (peroxisomes, mitochondria, perinuclear ER (pnER) or peripheral ER (pER), or fragments of the nucleus), that are subject to selective autophagy. The lower left panel depicts soluble selective autophagy receptors (SARs). The prApe1 dodecamer is bound by the coiled-coil (CC) domain of autophagy-related 19 (Atg19)58. Ams1 oligomerizes and associates with Atg19 through the Ams1-binding domain (ABD)116. prApe1, Ams1 and Atg19 assemble into a large complex called the cytoplasm-to-vacuole targeting (Cvt) complex. Atg34, an Atg19 paralogue, is also a receptor for Ams1 (REF. 24), but not for Ape1 or aspartyl aminopeptidase 4 (Ape4)117. Ape4 also binds Atg19. Coupling of ubiquitin conjugation to endoplasmic reticulum (ER) degradation 5 (Cue5) binds aggregates through direct interaction of its Cue domain with lysine 63 (K63)- and lysine 48(K48)-linked ubiquitin (Ub) chains that are covalently attached to cargoes25 by the E3 ubiquitin ligase Rsp5 and the E2 ubiquitin-conjugating enzyme Ubc4 or Ubc5. The lower right panel depicts membrane-associated SARs. Pexophagy receptors of Saccharomyces cerevisiae (Sc) Atg36 and Pichia pastoris (Pp) Atg30 recognize peroxisomal membrane proteins (Pex14 and/or Pex3). The mitophagy receptor Atg32 is embedded in the mitochondrial outer membrane via a single α-helical transmembrane domain (TMD) and probably (indicated by a question mark in the figure) the action of the tail-anchored (TA) mechanism, which refers to the protein machinery that inserts proteins possessing a carboxy-terminal TMD into the membrane such that, topologically, the amino terminus of the protein is cytosolic and the C terminus of the protein is lumenal. The ER-phagy receptors Atg39 and Atg40 have one TMD and two TMDs, respectively, and might insert into the ER membrane co-translationally via the signal recognition particle (SRP), the SRP receptor and the Sec61 translocon.