Summary
CD49d is a surface integrin that is expressed on chronic lymphocytic leukaemia (CLL) cells, and strongly correlates with more aggressive disease. Given its association with cell-cell adhesion and leucocyte trafficking, we hypothesized that patients with high CD49d expression would experience a clinical course dominated by lymphadenopathy. CD49d expression was measured by flow cytometry and considered positive if expressed by ≥30% of CLL cells. The study included 797 newly diagnosed CLL/small lymphocytic leukaemia patients; 279 (35%) were CD49d positive. CD49d-positive patients were more likely to present with lymphadenopathy (P < 0·001); a finding that persisted after adjusting for fluorescence in situ hybridisation (FISH) and IGHV mutation status [odds ratio (OR) 2·51; 95% confidence interval (CI) 1·64–3·83; P < 0·001]. Among CLL Rai 0 patients, CD49d positivity was associated with shorter time to development of lymphadenopathy (3·2 years vs not reached, P < 0·01). This association was maintained after adjusting for either FISH [hazard ratio (HR) 2·18; 95% CI 1·25–3·81; P = 0·006) or IGHV status (HR 2·02; 95% CI 1·11–3·69; P = 0·02) individually, but was attenuated when adjusting by both (HR 1·72; 95% CI 0·88–3·38; P = 0·11). These data demonstrate that CD49d-positive CLL patients experience a disease course dominated by lymphadenopathy. These findings could have implications for therapy selection and disease monitoring.
Keywords: chronic lymphocytic leukaemia, small lymphocytic lymphoma, CD49d, lymphadenopathy
Chronic lymphocytic leukaemia (CLL) is a clonal lymphoproliferative disorder characterized by a clonal B-cell population co-expressing CD5, CD19 and CD23, and weak expression of CD20, CD79b and surface immunoglobulin (Hallek et al, 2008). Patients with such a population who present with lymphadenopathy without lymphocytosis (<5 × 109/l) are designated as having small lymphocytic lymphoma (SLL) (Santos & O’Brien, 2012). Chronic lymphocytic leukaemia presents in heterogeneous ways, including asymptomatic lymphocytosis, B-symptoms (fever, night sweats, weight loss, fatigue), cytopenias due to marrow failure, or a lymph node predominant presentation. Although patients with deletion 11q23 and, to a lesser extent, trisomy 12 are at increased likelihood to experience lymphadenopathy, the biological factors that determine clinical presentation are largely unknown (Dohner et al, 1997; Strati et al, 2015).
CD49d, also known as integrin α4β1 (ITGB1), CD49d/CD29 or very late antigen 4 (VLA-4), is a surface integrin expressed on the surface of CLL lymphocytes that is essential for their grafting in animal models (Aydin et al, 2011). It is a strong independent predictor of survival in patients with CLL. High levels of CD49d protein and/or ITGB1 mRNA (Nuckel et al, 2009) are significantly associated with shorter time from diagnosis to treatment and overall survival (OS) (Zucchetto et al, 2005a,b, 2006). This prognostic value of CD49d is independent of other prognostic parameters, including fluorescence in situ hybridisation (FISH) and immunoglobulin heavy chain variable gene (IGHV) status (Gattei et al, 2008; Shanafelt et al, 2008), and is actually one of the strongest prognostic parameters identified to date (Bulian et al, 2014).
Given its association with cell-cell adhesion and leucocyte trafficking, we hypothesized that patients with high CD49d expression would experience a clinical course dominated by lymphadenopathy. Indeed, some studies have shown an association between high expression of CD49d and presentation as variant CLL (Cro et al, 2010), CLL with lymphadenopathies (Till et al, 2002), or SLL (Pinto et al, 1993). However, these series were small and nearly all the information to date is cross-sectional.
Here we present an analysis of the relationship between CD49d expression and the pattern of presentation and clinical course in a large cohort of newly diagnosed CLL patients.
Methods
Study population
This study was reviewed and approved by the Institutional Review Board of Mayo Clinic and was conducted in accordance with the principles of the Declaration of Helsinki. We identified all newly diagnosed CLL/SLL patients who were seen at Mayo Clinic within 12 months of diagnosis between August 2001 and December 2015. Analysis was limited to patients who had a pre-treated absolute B-cell count within 12 months of diagnosis and who underwent prognostic testing for CD49d.
Information regarding baseline clinical presentation, medical history, laboratory findings, and prognostic factors was obtained from clinical and research records. The latter included FISH for common CLL chromosome abnormalities, analysis of the IGHV mutation status and CD38, ZAP70 and CD49d expression by flow cytometry. CD49d was considered positive if expressed by ≥30% of cells, as previously described (Bulian et al, 2014).
Statistical analysis
To evaluate the association of CD49d with lymphadenopathy, we divided patients with Rai II–IV disease at diagnosis into 2 groups: Rai II–IV without lymphadenopathy or Rai II–IV with lymphadenopathy. To test for differences in CD49d (positive versus negative) cases by baseline characteristics, we used chi-square, rank sum, and trend tests where appropriate. A rank sum (Kruskal–Wallis) test for two-way comparisons was used to detect differences in CD49d expression levels by CLL presentation. A chi-square test was used to test the association of CD49d with presence of lymph nodes and presence of enlarged spleen at baseline. Multivariate logistic models, adjusted for IGHV mutation status and/or FISH, were used to test the association of CD49d status with presence of lymphadenopathy at diagnosis; odds ratios (OR) and 95% confidence intervals (CI) were computed.
We also evaluated time to development of lymphadenopathy based on CD49d status among patients who did not have lymphadenopathy at diagnosis. For this analysis, we plotted time to development of lymphadenopathy among patients who had Rai stage 0 disease at diagnosis using the cumulative incidence function, allowing for competing risks of treatment and death; Gray’s test was used to test differences between CD49d groups. Patients with Rai II–IV without lymphadenopathies were not included because of small sample (n = 30) and need for immediate treatment in many of these advanced stage patients (which eliminated the ability to evaluate the natural history with respect to lymphadenopathy). Multivariate Cox regression analysis, accounting for competing risks of treatment and death using Fine–Gray models, were used to test the association of CD49d and development of lymphadenopathy. Hazard ratios (HR) and 95% CI were computed. Analyses were run using SAS 9·4 (SAS Institute, Cary, NC, USA), and figures were created using R-3·1·1 (R Core Team, 2015).
Results
CD49d and presence of lymphadenopathy at diagnosis
The study included 797 patients: 386 patients with CLL Rai stage 0 disease, 137 with CLL Rai I, 30 with CLL Rai II–IV without lymphadenopathy, 62 with CLL Rai II–IV with lymphadenopathy and 182 patients with SLL. Two hundred and seventy-nine (35%) patients were CD49d positive. The remaining baseline characteristics are shown in Table I.
Table I.
Baseline characteristics of patients by CD49d status.
| Number (%), median [range] | ||||
|---|---|---|---|---|
|
|
||||
| Total N = 797 |
CD49d Positive n = 279 |
CD49d Negative n = 518 |
P-value | |
| Age (years) | 63 [24–93] | 65 [36–93] | 62 [24–93] | 0·027 |
| Males | 532 (67) | 199 (71) | 333 (64) | 0·044 |
| Females | 265 (33) | 80 (29) | 185 (36) | |
| Absolute lymphocyte count (× 109/l) | 12 [1–539] | 10 [1–539] | 13 [1–297] | <0·001 |
| Haemoglobin (g/l) | 139 [45–179] | 138 [45–179] | 140 [49–179] | 0·012 |
| Platelet count (× 109/l) | 195 [29–675] | 177 [41–469] | 201 [29–675] | <0·001 |
| CLL Rai 0 | 386 (48) | 74 (27) | 312 (60) | <0·001 |
| CLL Rai I | 137 (17) | 45 (16) | 92 (18) | |
| CLL Rai II–IV w/o LN | 30 (4) | 13 (5) | 17 (3) | |
| CLL Rai II–IV with LN | 62 (8) | 31 (11) | 31 (6) | |
| SLL | 182 (23) | 116 (42) | 66 (13) | |
| Baseline LN | 381 | 192 (69) | 189 (36) | <0·001 |
| No baseline LN | 416 | 87 (31) | 329 (64) | |
| CD38 negative | 552 (72) | 114 (44) | 438 (87) | <0·001 |
| Positive | 215 (28) | 148 (56) | 67 (13) | |
| Missing | 30 | 17 | 13 | |
| ZAP70 negative | 455 (60) | 117 (44) | 338 (68) | <0·001 |
| Positive | 304 (40) | 148 (56) | 156 (32) | |
| Missing | 38 | 14 | 24 | |
| IGHV mutated | 356 (53) | 79 (35) | 277 (63) | <0·001 |
| Unmutated | 312 (47) | 149 (65) | 163 (37) | |
| Missing | 129 | 51 | 78 | |
| FISH Del13q | 315 (44) | 52 (21) | 263 (55) | <0·001 |
| Normal | 182 (25) | 57 (23) | 125 (26) | |
| +12 | 115 (16) | 100 (41) | 15 (3) | |
| Del11q | 68 (9) | 15 (6) | 53 (11) | |
| Del17p | 30 (4) | 14 (6) | 16 (3) | |
| Other | 13 (2) | 5 (2) | 8 (2) | |
| Missing | 74 | 36 | 38 | |
CLL, chronic lymphocytic leukaemia; FISH, fluorescence in situ hybridisation; LN, lymphadenopathy; SLL, small lymphocytic leukaemia; w/o, without.
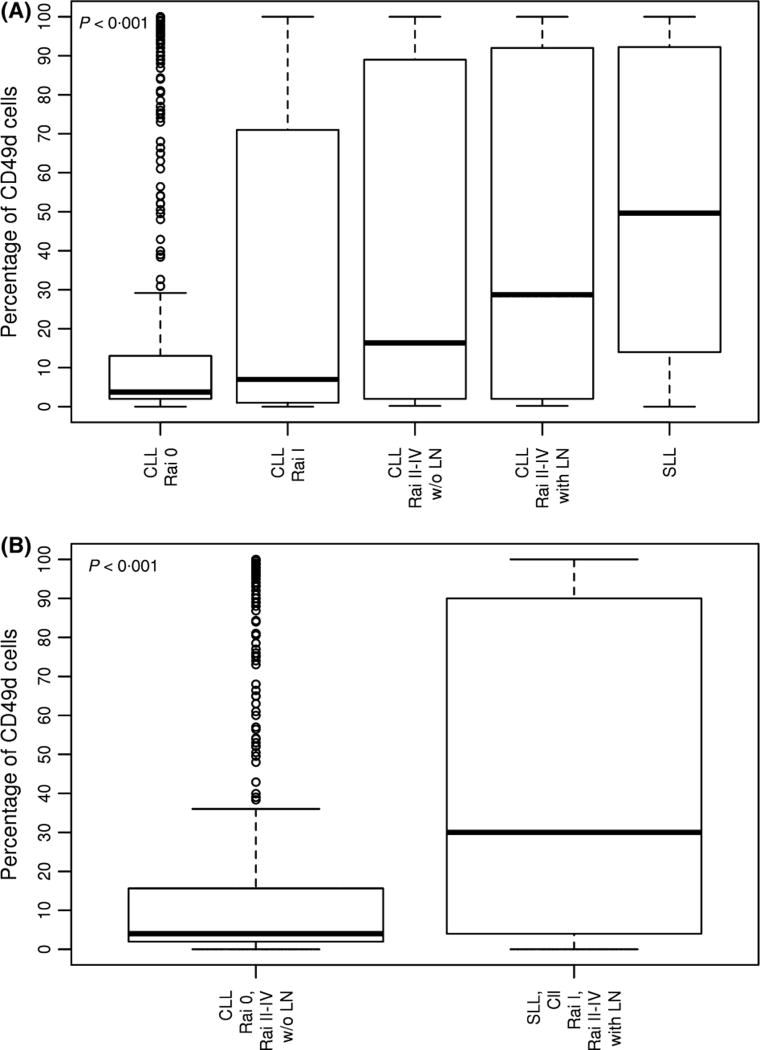
The median percentage of leukaemic cells expressing CD49d at diagnosis varied by stage and was lowest among patients with Rai 0 disease and highest among those with SLL (P < 0·001; Fig 1A). Compared to CLL Rai 0, a higher percentage was observed for CLL Rai II–IV without lymphadenopathy (P = 0·01), CLL Rai II–IV with lymphadenopathy (P < 0·001) and SLL (P < 0·001), but not for CLL Rai I (P = 0·11). When patients were categorized by the presence (Rai I, Rai II–IV with lymphadenopathy, SLL) or absence (Rai 0 and Rai II–IV without lymphadenopathy) of lymphadenopathy at diagnosis, a significantly higher percentage of leukaemic cells expressing CD49d was observed among patients presenting with lymphadenopathy (P < 0·001; Fig 1B).
Fig 1.
Association between CD49d expression at time of diagnosis and type of CLL presentation. The percentage of lymphocytes expressing CD49d is shown on the y-axis (each bar shows median, first, and third quartiles). (A) By Rai stage; (B) By lymphadenopathy. CLL, chronic lymphocytic leukaemia; LN, lymphadenopathy; SLL, small lymphocytic leukaemia; w/o, without.
Baseline lymphadenopathy was more frequent among CD49d-positive patients (69%) than CD49d-negative patients (36%) (P < 0·001). Baseline lymphadenopathy was also more frequent among patients with trisomy 12 as compared to patients without trisomy 12 (67% vs. 41%, P < 0·001), among patients with deletion 11q as compared to patients without deletion 11q (64% vs. 43%, P < 0·001) and among patients with unmutated IGHV as compared to patients with mutated IGHV (63% vs. 33%, P < 0·001). The association between baseline lymphadenopathy and CD49d positivity was maintained on multivariate analyses after adjusting for FISH and IGHV status (OR = 2·51; 95% CI 1·64–3·83; P < 0·001).
Interestingly, baseline splenomegaly was more frequent among CD49d-positive patients (15%) than among CD49dnegative patients (8%) (P = 0·003).
CD49d and development of lymphadenopathy among Rai 0 Patients
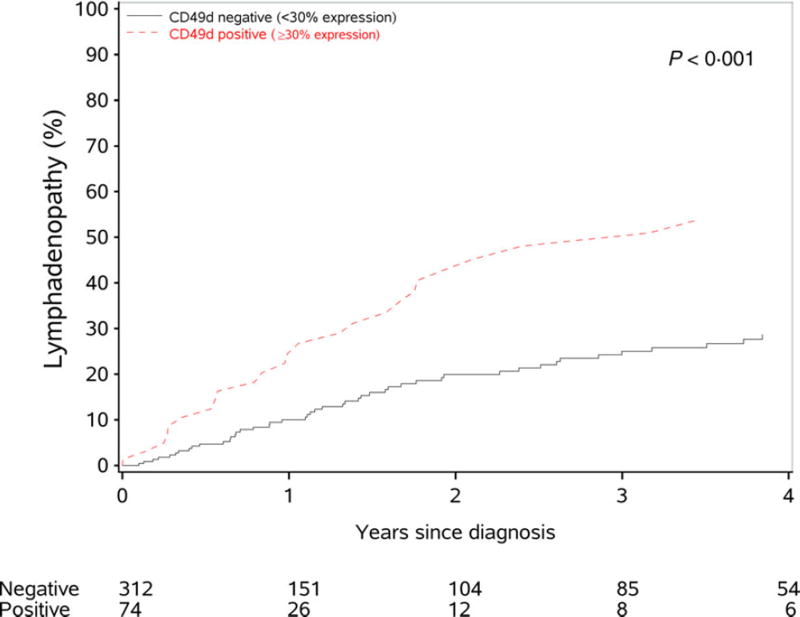
We next evaluated the association between baseline CD49d status and subsequent development of lymphadenopathy among the 386 patients with CLL Rai stage 0 disease at the time of diagnosis. Eighty-two patients with CLL Rai 0 developed palpable lymphadenopathy during the course of follow-up (median time to development of lymphadenopathy: not reached). CD49d status at baseline was strongly associated with development of lymphadenopathy among patients with Rai stage 0 disease at diagnosis (median time to lymphadenopathy: 3·2 years in CD49d-positive patients vs not reached in CD49d-negative patients; HR 2·44, P < 0·01) (Fig 2). The association was maintained after adjusting for either FISH (HR 2·18; 95% CI 1·25–3·81; P = 0·006) or IGHV status (HR 2·02; 95% CI 1·11–3·69; P = 0·02), but was attenuated after adjusting for both at the same time (HR 1·72; 95% CI 0·88–3·38; P = 0·11).
Fig 2.
Time from diagnosis to lymphadenopathy development by CD49d negative (<30% expression), CD49d positive (>30% expression) among newly diagnosed chronic lymphocytic leukaemia patients presenting with Rai stage 0 disease.
Discussion
The clinical course of some CLL patients is dominated by lymphadenopathy whereas lymphocytosis, B-symptoms or cytopenias are characteristic in others. Other than an association with the genetic defects deletion 11q23 and trisomy 12, the biological characteristics associated with lymphadenopathy are unknown. Here, we demonstrate that CD49d expression is strongly related to the presence of lymphadenopathy at the time of diagnosis as well as development of lymphadenopathy during the course of the disease.
It should be noted that the expression of CD49d correlates with some other prognostic factors. Specifically, higher expression of CD49d is associated with unmutated IGHV (Sulda et al, 2012), IGHV3-21 (Bomben et al, 2007), CD38 (Pittner et al, 2005) and ZAP70 (Degheidy et al, 2011). Notably, CD49d expression is typically lower among patients with deletion 11q (Sembries et al, 1999) despite the association of this genetic defect with lymphadenopathy. In contrast, CD49d expression is higher among patients with trisomy 12, probably facilitated through a NOTCH1 (Riches et al, 2014) or methylation-mediated mechanism (Zucchetto et al, 2013). In our cohort, the association between CD49d expression and lymphadenopathy at the time of CLL diagnosis was maintained also after adjusting for FISH and IGHV status, confirming its independent role. In addition, among patients with Rai stage 0, CD49d was also associated with subsequent development of lymphadenopathy, independently of either FISH or IGHV status.
Given the role CD49d plays in lymphocyte adhesion and trafficking, there is a strong biological underpinning to these findings. Previous studies have in fact demonstrated that CLL cells collected from lymph nodes have higher CD49d expression than those in the peripheral blood (Pasikowska et al, 2016). CD49d interacts with the CLL microenvironment, including bone marrow stroma (Plander et al, 2011), fibroblasts (Hamilton et al, 2012) and vascular endothelial cells (Buggins et al, 2010). This is coordinated by chemokines (e.g., CCL3 and CCL4), adhesion molecules [e.g. VCAM (Zucchetto et al, 2009)], chemokine receptors [e.g., CXCR4 (Majid et al, 2011)], and metalloproteinases [e.g., MMP9(Redondo-Munoz et al, 2008)]. CD49d also interacts with CLL-embedded molecules, such as CD38 (Zucchetto et al, 2012), ZAP70, the B-cell receptor (Calpe et al, 2011), CD26 (Cro et al, 2009), CD44 (Buggins et al, 2011) and AID (Palacios et al, 2010). Of interest, it doesn’t interact with DPP4 (Sulda et al, 2010), and its biological effect may be subsidized by the c-met receptor (Eksioglu-Demiralp et al, 2011). In small and cross-sectional series, CD49d was associated with high disease burden (Lucio et al, 1998) and advanced Rai stage (Eksioglu-Demiralp et al, 1996) but not with splenomegaly (Bairey et al, 2004). The association between CD49d expression and positivity and initial presentation with lymphadenopathy or splenomegaly has been confirmed in our study, the largest series published to date.
In addition to new biological questions, the strong relationship between CD49d expression and lymphadenopathy raises several clinical crucial questions. While baseline computed tomography (CT) imaging is standard of care for most sub-types of low grade non-Hodgkin lymphoma, it is typically not recommended in patients with newly diagnosed CLL patients. The strong relationship between CD49d and lymphadenopathy suggests that the role of imaging in the monitoring of CD49d positive CLL patients should be explored. It also raises the question of whether baseline CT imaging should be pursued in the subset of individuals with clinical monoclonal B-cell lymphocytosis who are CD49d positive, as the likelihood they would be reclassified as SLL may be high. Further investigation of these aspects is needed before any change in clinical practice can be recommended. Finally, CD49d may play a predictive role, particularly in patients with lymphadenopathy; at present, five drugs have shown the ability to decrease CD49d expression and/or inhibit CD49d function, namely Ibrutinib (Pepper et al, 2015), Fosamatinib (Buchner et al, 2010), Idelalisib (Fiorcari et al, 2013), Natalizumab (Walsby et al, 2014), and OSU-T315 (Liu et al, 2015). The effects of these drugs on the biology of nodal disease and their impact on CD49d expression should be explored.
In conclusion, CD49d expression was associated with nodal disease at the time of presentation in patients with newly diagnosed CLL, as well as with the development of lymphadenopathy among those initially presenting with CLL Rai stage 0 disease. Additional studies are needed to determine the biological underpinnings of this observation and its potential implications for the role of imaging in disease monitoring in newly diagnosed CLL patients.
Acknowledgments
This study was funded by the Cancer Center Grant and R01CA197120 (PI Shanafelt). Sameer A. Parikh is a recipient of the K12 CA090628 grant from the National Cancer Institute.
Parikh: Research funding (Pharmacyclics); Advisory Boards (Pharmacyclics).
Footnotes
Author contributions
TDS designed the study, analysed the data, provided clinical care to patients and wrote the paper; PS designed the study, analysed data and wrote the paper; TGC, SAP, WD and NEK provided clinical care to patients and co-authored the paper; KGC, SJA, and SLS collected and analysed the data and coauthored the paper; CAH and DFJ provided pathology and flow cytometry data.
Conflict of interest
The other authors declare no conflicts of interest.
References
- Aydin S, Grabellus F, Eisele L, Mollmann M, Hanoun M, Ebeling P, Moritz T, Carpinteiro A, Nuckel H, Sak A, Gothert JR, Duhrsen U, Durig J. Investigating the role of CD38 and functionally related molecular risk factors in the CLL NOD/SCID xenograft model. European Journal of Haematology. 2011;87:10–19. doi: 10.1111/j.1600-0609.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- Bairey O, Zimra Y, Rabizadeh E, Shaklai M. Expression of adhesion molecules on leukemic B cells from chronic lymphocytic leukemia patients with predominantly splenic manifestations. The Israel Medical Association Journal: IMAJ. 2004;6:147–151. [PubMed] [Google Scholar]
- Bomben R, Dal Bo M, Capello D, Benedetti D, Marconi D, Zucchetto A, Forconi F, Maffei R, Ghia EM, Laurenti L, Bulian P, Del Principe MI, Palermo G, Thorselius M, Degan M, Campanini R, Guarini A, Del Poeta G, Rosenquist R, Efremov DG, Marasca R, Foa R, Gaidano G, Gattei V. Comprehensive characterization of IGHV3-21-expressing B-cell chronic lymphocytic leukemia: an Italian multicenter study. Blood. 2007;109:2989–2998. doi: 10.1182/blood-2006-10-051110. [DOI] [PubMed] [Google Scholar]
- Buchner M, Baer C, Prinz G, Dierks C, Burger M, Zenz T, Stilgenbauer S, Jumaa H, Veelken H, Zirlik K. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115:4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- Buggins AG, Pepper C, Patten PE, Hewamana S, Gohil S, Moorhead J, Folarin N, Yallop D, Thomas NS, Mufti GJ, Fegan C, Devereux S. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Research. 2010;70:7523–7533. doi: 10.1158/0008-5472.CAN-10-1634. [DOI] [PubMed] [Google Scholar]
- Buggins AG, Levi A, Gohil S, Fishlock K, Patten PE, Calle Y, Yallop D, Devereux S. Evidence for a macromolecular complex in poor prognosis CLL that contains CD38, CD49d, CD44 and MMP-9. British Journal of Haematology. 2011;154:216–222. doi: 10.1111/j.1365-2141.2011.08725.x. [DOI] [PubMed] [Google Scholar]
- Bulian P, Shanafelt TD, Fegan C, Zucchetto A, Cro L, Nuckel H, Baldini L, Kurtova AV, Ferrajoli A, Burger JA, Gaidano G, Del Poeta G, Pepper C, Rossi D, Gattei V. CD49d is the strongest flow cytometry-based predictor of overall survival in chronic lymphocytic leukemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2014;32:897–904. doi: 10.1200/JCO.2013.50.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calpe E, Codony C, Baptista MJ, Abrisqueta P, Carpio C, Purroy N, Bosch F, Crespo M. ZAP-70 enhances migration of malignant B lymphocytes toward CCL21 by inducing CCR7 expression via IgM-ERK1/2 activation. Blood. 2011;118:4401–4410. doi: 10.1182/blood-2011-01-333682. [DOI] [PubMed] [Google Scholar]
- Cro L, Morabito F, Zucal N, Fabris S, Lionetti M, Cutrona G, Rossi F, Gentile M, Ferrario A, Ferrarini M, Molica S, Neri A, Baldini L. CD26 expression in mature B-cell neoplasia: its possible role as a new prognostic marker in B-CLL. Hematological Oncology. 2009;27:140–147. doi: 10.1002/hon.888. [DOI] [PubMed] [Google Scholar]
- Cro L, Ferrario A, Lionetti M, Bertoni F, Zucal NN, Nobili L, Fabris S, Todoerti K, Cortelezzi A, Guffanti A, Goldaniga M, Marcheselli L, Neri A, Lambertenghi-Deliliers G, Baldini L. The clinical and biological features of a series of immunophenotypic variant of B-CLL. European Journal of Haematology. 2010;85:120–129. doi: 10.1111/j.1600-0609.2010.01454.x. [DOI] [PubMed] [Google Scholar]
- Degheidy HA, Venzon DJ, Farooqui MZ, Abbasi F, Arthur DC, Wilson WH, Wiestner A, Stetler-Stevenson MA, Marti GE. Improved ZAP-70 assay using two clones, multiple methods of analysis and clinical correlation. Cytometry. Part B, Clinical Cytometry. 2011;80:309–317. doi: 10.1002/cyto.b.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner H, Stilgenbauer S, James MR, Benner A, Weilguni T, Bentz M, Fischer K, Hunstein W, Lichter P. 11q deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood. 1997;89:2516–2522. [PubMed] [Google Scholar]
- Eksioglu-Demiralp E, Alpdogan O, Aktan M, Firatli T, Ozturk A, Budak T, Bayik M, Akoglu T. Variable expression of CD49d antigen in B cell chronic lymphocytic leukemia is related to disease stages. Leukemia. 1996;10:1331–1339. [PubMed] [Google Scholar]
- Eksioglu-Demiralp E, Akdeniz T, Bayik M. Aberrant expression of c-met and HGF/c-met pathway provides survival advantage in B-chronic lymphocytic leukemia. Cytometry. Part B, Clinical Cytometry. 2011;80:1–7. doi: 10.1002/cyto.b.20553. [DOI] [PubMed] [Google Scholar]
- Fiorcari S, Brown WS, McIntyre BW, Estrov Z, Maffei R, O’Brien S, Sivina M, Hoellenriegel J, Wierda WG, Keating MJ, Ding W, Kay NE, Lannutti BJ, Marasca R, Burger JA. The PI3-kinase delta inhibitor idelalisib (GS-1101) targets integrin-mediated adhesion of chronic lymphocytic leukemia (CLL) cell to endothelial and marrow stromal cells. PLoS ONE. 2013;8:e83830. doi: 10.1371/journal.pone.0083830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattei V, Bulian P, Del Principe MI, Zucchetto A, Maurillo L, Buccisano F, Bomben R, Dal-Bo M, Luciano F, Rossi FM, Degan M, Amadori S, Del Poeta G. Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood. 2008;111:865–873. doi: 10.1182/blood-2007-05-092486. [DOI] [PubMed] [Google Scholar]
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton E, Pearce L, Morgan L, Robinson S, Ware V, Brennan P, Thomas NS, Yallop D, Devereux S, Fegan C, Buggins AG, Pepper C. Mimicking the tumour microenvironment: three different co-culture systems induce a similar phenotype but distinct proliferative signals in primary chronic lymphocytic leukaemia cells. British Journal of Haematology. 2012;158:589–599. doi: 10.1111/j.1365-2141.2012.09191.x. [DOI] [PubMed] [Google Scholar]
- Liu TM, Ling Y, Woyach JA, Beckwith K, Yeh YY, Hertlein E, Zhang X, Lehman A, Awan F, Jones JA, Andritsos LA, Maddocks K, MacMurray J, Salunke SB, Chen CS, Phelps MA, Byrd JC, Johnson AJ. OSU-T315: a novel targeted therapeutic that antagonizes AKT membrane localization and activation of chronic lymphocytic leukemia cells. Blood. 2015;125:284–295. doi: 10.1182/blood-2014-06-583518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucio PJ, Faria MT, Pinto AM, da Silva MR, Correia Junior ME, da Costa RJ, Parreira AB. Expression of adhesion molecules in chronic B-cell lymphoproliferative disorders. Haematologica. 1998;83:104–111. [PubMed] [Google Scholar]
- Majid A, Lin TT, Best G, Fishlock K, Hewamana S, Pratt G, Yallop D, Buggins AG, Wagner S, Kennedy BJ, Miall F, Hills R, Devereux S, Oscier DG, Dyer MJ, Fegan C, Pepper C. CD49d is an independent prognostic marker that is associated with CXCR4 expression in CLL. Leukemia Research. 2011;35:750–756. doi: 10.1016/j.leukres.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Nuckel H, Switala M, Collins CH, Sellmann L, Grosse-Wilde H, Duhrsen U, Rebmann V. High CD49d protein and mRNA expression predicts poor outcome in chronic lymphocytic leukemia. Clinical Immunology. 2009;131:472–480. doi: 10.1016/j.clim.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Palacios F, Moreno P, Morande P, Abreu C, Correa A, Porro V, Landoni AI, Gabus R, Giordano M, Dighiero G, Pritsch O, Oppezzo P. High expression of AID and active class switch recombination might account for a more aggressive disease in unmutated CLL patients: link with an activated microenvironment in CLL disease. Blood. 2010;115:4488–4496. doi: 10.1182/blood-2009-12-257758. [DOI] [PubMed] [Google Scholar]
- Pasikowska M, Walsby E, Apollonio B, Cuthill K, Phillips E, Coulter E, Longhi MS, Ma Y, Yallop D, Barber LD, Patten P, Fegan C, Ramsay AG, Pepper C, Devereux S, Buggins AG. Phenotype and immune function of lymph node and peripheral blood CLL cells are linked to transendothelial migration. Blood. 2016;128:563–573. doi: 10.1182/blood-2016-01-683128. [DOI] [PubMed] [Google Scholar]
- Pepper C, Buggins AG, Jones CH, Walsby EJ, Forconi F, Pratt G, Devereux S, Stevenson FK, Fegan C. Phenotypic heterogeneity in IGHV-mutated CLL patients has prognostic impact and identifies a subset with increased sensitivity to BTK and PI3Kdelta inhibition. Leukemia. 2015;29:744–747. doi: 10.1038/leu.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Carbone A, Gloghini A, Marotta G, Volpe R, Zagonel V. Differential expression of cell adhesion molecules in B-zone small lymphocytic lymphoma and other well-differentiated lymphocytic disorders. Cancer. 1993;72:894–904. doi: 10.1002/1097-0142(19930801)72:3<894::aid-cncr2820720339>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Pittner BT, Shanafelt TD, Kay NE, Jelinek DF. CD38 expression levels in chronic lymphocytic leukemia B cells are associated with activation marker expression and differential responses to interferon stimulation. Leukemia. 2005;19:2264–2272. doi: 10.1038/sj.leu.2403975. [DOI] [PubMed] [Google Scholar]
- Plander M, Ugocsai P, Seegers S, Orso E, Reichle A, Schmitz G, Hofstadter F, Brockhoff G. Chronic lymphocytic leukemia cells induce anti-apoptotic effects of bone marrow stroma. Annals of Hematology. 2011;90:1381–1390. doi: 10.1007/s00277-011-1218-z. [DOI] [PubMed] [Google Scholar]
- R Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2015. R: A language and environment for statistical computing. URL https://www.R-project.org/ [Google Scholar]
- Redondo-Munoz J, Ugarte-Berzal E, Garcia-Marco JA, del Cerro MH, Van den Steen PE, Opdenakker G, Terol MJ, Garcia-Pardo A. Alpha4beta1 integrin and 190-kDa CD44v constitute a cell surface docking complex for gelatinase B/MMP-9 in chronic leukemic but not in normal B cells. Blood. 2008;112:169–178. doi: 10.1182/blood-2007-08-109249. [DOI] [PubMed] [Google Scholar]
- Riches JC, O’Donovan CJ, Kingdon SJ, McClanahan F, Clear AJ, Neuberg DS, Werner L, Croce CM, Ramsay AG, Rassenti LZ, Kipps TJ, Gribben JG. Trisomy 12 chronic lymphocytic leukemia cells exhibit upregulation of integrin signaling that is modulated by NOTCH1 mutations. Blood. 2014;123:4101–4110. doi: 10.1182/blood-2014-01-552307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos FP, O’Brien S. Small lymphocytic lymphoma and chronic lymphocytic leukemia: are they the same disease? Cancer Journal. 2012;18:396–403. doi: 10.1097/PPO.0b013e31826cda2d. [DOI] [PubMed] [Google Scholar]
- Sembries S, Pahl H, Stilgenbauer S, Dohner H, Schriever F. Reduced expression of adhesion molecules and cell signaling receptors by chronic lymphocytic leukemia cells with 11q deletion. Blood. 1999;93:624–631. [PubMed] [Google Scholar]
- Shanafelt TD, Geyer SM, Bone ND, Tschumper RC, Witzig TE, Nowakowski GS, Zent CS, Call TG, Laplant B, Dewald GW, Jelinek DF, Kay NE. CD49d expression is an independent predictor of overall survival in patients with chronic lymphocytic leukaemia: a prognostic parameter with therapeutic potential. British Journal of Haematology. 2008;140:537–546. doi: 10.1111/j.1365-2141.2007.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati P, Abruzzo LV, Wierda WG, O’Brien S, Ferrajoli A, Keating MJ. Second cancers and Richter transformation are the leading causes of death in Patients with trisomy 12 chronic lymphocytic leukemia. Clinical Lymphoma Myeloma & Leukemia. 2015;15:420–427. doi: 10.1016/j.clml.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulda ML, Abbott CA, Macardle PJ, Hall RK, Kuss BJ. Expression and prognostic assessment of dipeptidyl peptidase IV and related enzymes in B-cell chronic lymphocytic leukemia. Cancer Biology & Therapy. 2010;10:180–189. doi: 10.4161/cbt.10.2.12168. [DOI] [PubMed] [Google Scholar]
- Sulda ML, Kuss BJ, Hall RK, Bailey S, Macardle PJ. Clinical utility of molecular and flow cytometric markers in chronic lymphocytic leukaemia. Internal Medicine Journal. 2012;42:137–146. doi: 10.1111/j.1445-5994.2010.02294.x. [DOI] [PubMed] [Google Scholar]
- Till KJ, Lin K, Zuzel M, Cawley JC. The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99:2977–2984. doi: 10.1182/blood.v99.8.2977. [DOI] [PubMed] [Google Scholar]
- Walsby E, Buggins A, Devereux S, Jones C, Pratt G, Brennan P, Fegan C, Pepper C. Development and characterization of a physiologically relevant model of lymphocyte migration in chronic lymphocytic leukemia. Blood. 2014;123:3607–3617. doi: 10.1182/blood-2013-12-544569. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Sonego P, Degan M, Bomben R, Dal Bo M, Russo S, Attadia V, Rupolo M, Buccisano F, Del Principe MI, Del Poeta G, Pucillo C, Colombatti A, Campanini R, Gattei V. Signature of B-CLL with different prognosis by Shrunken centroids of surface antigen expression profiling. Journal of Cellular Physiology. 2005a;204:113–123. doi: 10.1002/jcp.20269. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Sonego P, Degan M, Bomben R, Dal Bo M, Russo S, Attadia V, Rupolo M, Buccisano F, Steffan A, Del Poeta G, Pucillo C, Colombatti A, Campanini R, Gattei V. Surface-antigen expression profiling (SEP) in B-cell chronic lymphocytic leukemia (B-CLL): Identification of markers with prognostic relevance. Journal of Immunological Methods. 2005b;305:20–32. doi: 10.1016/j.jim.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Bomben R, Dal Bo M, Sonego P, Nanni P, Rupolo M, Bulian P, Dal Maso L, Del Poeta G, Del Principe MI, Degan M, Gattei V. A scoring system based on the expression of six surface molecules allows the identification of three prognostic risk groups in B-cell chronic lymphocytic leukemia. Journal of Cellular Physiology. 2006;207:354–363. doi: 10.1002/jcp.20570. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Benedetti D, Tripodo C, Bomben R, Dal Bo M, Marconi D, Bossi F, Lorenzon D, Degan M, Rossi FM, Rossi D, Bulian P, Franco V, Del Poeta G, Deaglio S, Gaidano G, Tedesco F, Malavasi F, Gattei V. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Research. 2009;69:4001–4009. doi: 10.1158/0008-5472.CAN-08-4173. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Vaisitti T, Benedetti D, Tissino E, Bertagnolo V, Rossi D, Bomben R, Dal Bo M, Del Principe MI, Gorgone A, Pozzato G, Gaidano G, Del Poeta G, Malavasi F, Deaglio S, Gattei V. The CD49d/CD29 complex is physically and functionally associated with CD38 in B-cell chronic lymphocytic leukemia cells. Leukemia. 2012;26:1301–1312. doi: 10.1038/leu.2011.369. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Caldana C, Benedetti D, Tissino E, Rossi FM, Hutterer E, Pozzo F, Bomben R, Dal Bo M, D’Arena G, Zaja F, Pozzato G, Di Raimondo F, Hartmann TN, Rossi D, Gaidano G, Del Poeta G, Gattei V. CD49d is overexpressed by trisomy 12 chronic lymphocytic leukemia cells: evidence for a methylation-dependent regulation mechanism. Blood. 2013;122:3317–3321. doi: 10.1182/blood-2013-06-507335. [DOI] [PubMed] [Google Scholar]