Figure 1.
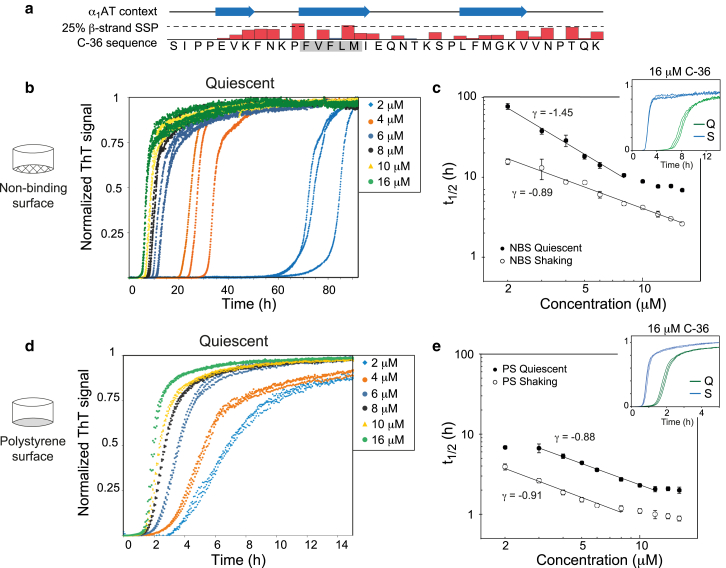
Surface type profoundly affects C-36 fibrillation kinetics and alters the balance between primary and secondary nucleation. (a) Given here is an overview of the C-36 peptide with indication of the secondary structure in the context of α1AT and the weak β-strand secondary structural propensity (SSP) in solution determined by liquid-state NMR (adapted from (42)). The aggregation-prone FVFLM is boxed in gray. (b) Kinetic traces are displayed in triplicates for C-36 under quiescent conditions carried out in NBS. (c) Double logarithmic plots show the time to half-completion (t1/2) ± SD under quiescent or shaking conditions (300 RPM) as a function of C-36 concentration. The scaling relationship for indicated linear fits with SE is γ = −1.45 ± 0.09 for quiescent conditions and γ = −0.89 ± 0.04 for shaking. (d) Kinetic traces are displayed in triplicates for C-36 under quiescent conditions carried out in polystyrene surface plates (PS). (e) Double logarithmic plots of the time to half completion (t1/2) ± SD under quiescent or shaking conditions (300 RPM) are given as a function of C-36 concentration. The scaling relationship for indicated linear fits with SE is γ = −0.88 ± 0.03 for quiescent conditions and γ = −0.91 ± 0.03 for shaking. Insets in (c) and (e) compare kinetic traces for 16 μM C-36 under shaking (S) and quiescent (Q) conditions. To see this figure in color, go online.