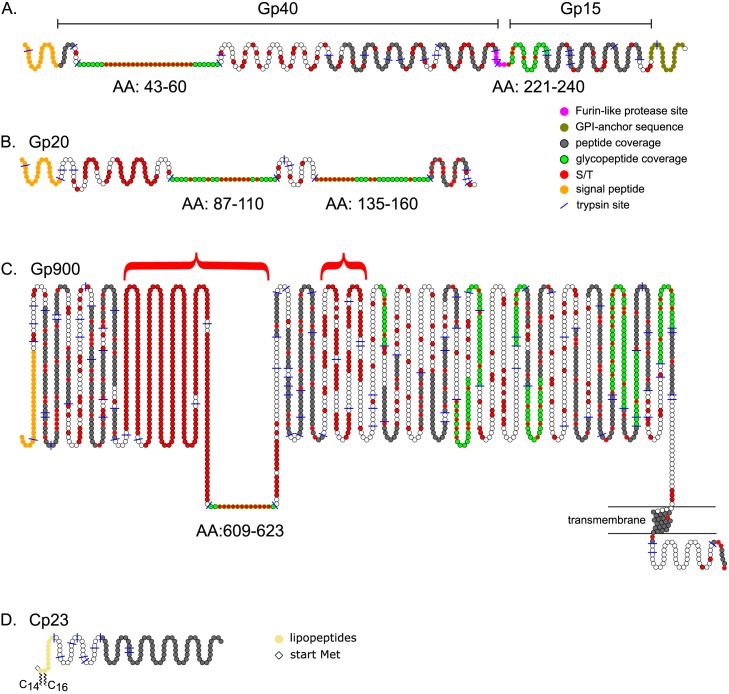
Fig 1. Schematics of Cryptosporidium glycoproteins characterized here by mass spectrometry.
(A) Gp40/Gp15 precursor is cleaved at a furin-like protease site (pink) into Gp40 and Gp15. Mass spectrometry showed Gp40 has a Thr-rich domain (AA-43 to 60) with numerous O-linked HexNAc modifications (marked in green, with Ser and Thr residues marked in red). Gp15 contains a single domain (AA-221 to 240) that is glycosylated. Other peptides identified with mass spectrometry are marked in grey. Predicted N-terminal signal peptide is marked in orange, while GPI-anchor signal is marked in olive. (B) A 20-kDa glycoprotein (Gp20) contains two Thr-rich domains (AA-87 to110 and AA-135 to 160), which contain numerous HexNAc modifications. (C) Gp900 contains two very large Thr-rich domains (red brackets), one of which contains a peptide with three HexNAc residues (AA-609 to 623). The transmembrane helix near the C-terminus is encompassed by two horizontal lines, representing a membrane. (D) The N-terminus of Cp23 is modified with N-myristate (C14) and S-palmitate (C16). The start Met is absent (diamond).