Abstract
The aim of this study was to compare the effect of dietary supplementation with low dose of porous and nano zinc oxide (ZnO) on weaning piglets, and to evaluate the possibility of using them as an alternative to high dose of regular ZnO. Piglets were randomly allocated into four treatment groups fed with four diets: (1) basal diet (NC), (2) NC+ 3000 mg/kg ZnO (PC), (3) NC + 500 mg/kg porous ZnO (HiZ) and (4) NC + 500 mg/kg nano ZnO (ZNP). The result showed that piglets in HiZ group had less diarrhea than ZNP group (P < 0.05). Besides, there was no significant difference between PC, HiZ and ZNP groups in terms of serum malondialdeyhde (MDA) concentration and glutathione peroxidase (GSH-Px) activity (P > 0.05). Analysis of trace metal elements revealed that piglets fed with high dose of regular ZnO had the highest Zn level in kidney (P < 0.05), which may induce kidney stone formation. Additionally, a decrease in ileal crypt depth was observed in PC, HiZ and ZNP group, suggesting an effective protection against intestinal injury. Results of mRNA analysis in intestine showed that ZNP supplementation had better effects on up-regulated trefoil factor 3 (TFF3) and nuclear factor erythroid 2-related factor 2 (Nrf2) levels in duodenum and jejunum than HiZ did (P < 0.05), implying that nano ZnO may possess higher anti-inflammatory capacity than porous ZnO. In conclusion, dietary supplementation with low dose of porous and nano ZnO had similar (even better) effect on improving growth performance and intestinal morphology, reducing diarrhea and intestinal inflammatory as high dose of regular ZnO in weaning piglets. Compared with nano ZnO, porous ZnO had better performance on reducing diarrhea but less effect on up-regulation of intestinal TFF3 and Nrf2.
Introduction
Zinc oxide (ZnO) is a multifunctional material because of its diverse properties [1]. It plays an important role in a very wide range of applications, ranging from ceramics to tyres [2], from pharmaceuticals to agriculture [3, 4], and from chemicals to dye[5, 6]. Due to its antibacterial, disinfecting and drying properties, ZnO is widely used in the production of medicines for epilepsy [7], diarrhea [8] and wound healing[9]. At the present time, the advent of nanotechnology has brought great opportunities for the development of ZnO in many areas. ZnO in nanoscale has shown potential applications in food preservation [3], cancer control [10, 11] and anti-viral treatment [12].
In swine production, the characteristic of ZnO in diarrhea prevention has drawn a great attention. Post-weaning diarrhea is one of the most common causes of morbidity and mortality for weaning piglets, resulting in reduced growth performance in piglets [13]. During the first two weeks after weaning, the gastrointestinal tract of piglets undergoes a dynamic stress process, likely due to nutritional, psychological, environmental and physiological factors [14]. In 1980s, it was found that feeding with pharmacological concentrations of ZnO (2000 to 4000 mg/kg) can reduce diarrhea and increase growth rates in weanling piglets [15]. As the antimicrobial growth promoters being banned in many countries, dietary high dose of ZnO have drawn a great concerns from researchers and farm workers [16–18].
Recently, some disadvantages have been found when high dose of ZnO were extensively uses in pig production. Bednorz et al. [19] found that feeding with ZnO at the dose of 2500 ppm increases the proportion of multi-resistant Escherichia coli in ileum and colon digesta. It provokes the question about the reasonability of high-level Zn supplementation as a result of the ban of antimicrobial growth promoters. On the other side, the bioavailability of Zn from ZnO is lower than that from other Zn sources, such as ZnSO4, Zn-methionine and Zn-lysine [20]. Feeding piglets with high dose of ZnO results in plenty of un-absorbable Zn being released to environment, finally increasing the risks of multiple dug resistance [19] and heavy metal contamination [21]. Thus, some researchers attempted to reduce the usage of ZnO in pig feed and enhance the biological effect of ZnO (especially on diarrhea). At the present time, changing regular ZnO powder into porous particles or nanoparticles is a common practice [22–24]. Morales et al. [23] found that low dose of porous ZnO (HiZox, 150 ppm) significantly improved the growth performance and health status of piglets compared to pharmacological dose of regular ZnO in the stater phase. On the other side, Cho et al. [25] found that compared with TiO2 nanoparticles, ZnO nanoparticles had higher absorption and more extensive organ distribution when administered orally in rat, suggesting it is a potential alternative form of regular ZnO powder. Thus, both porous ZnO and nano ZnO were deemed as potential candidates for replacing high-dose dietary regular ZnO in weaning piglets. Further evaluation of the tissue distribution, diarrhea prevention, anti-inflammatory effect of these ZnO products following oral administration in piglets is still needed to provide valuable information for assessing the merits of these particles. Therefore, we compared the effects of porous and nano ZnO on growth performance, diarrhea, tissue Zn distribution, blood biochemistry parameters, intestinal morphology and inflammation in weaning piglets. The possibility of replacing high-dose dietary regular ZnO with low-dose porous and nano ZnO was also assessed in this study.
Materials and methods
Animals and experimental design
This study was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving animal subjects were approved by the animal welfare committee of the Institute of Subtropical Agriculture, Chinese Academy of Science.
A total of 128 piglets (Duroc× Landrace × Yorkshire, weaned at 35 ± 1 d with BW of 10.96 ± 1.25 kg) were used in a 28-d experiment. They were randomly allotted to four dietary treatments. Dietary treatments were replicated using eight pens (four piglets per pen, two males and two females). The experimental diets were formulated using corn and soybean meal supplemented with three forms of ZnO: regular powder, porous particles and nanoparticles. Porous ZnO (HiZox) was kindly offered by Animine Co., Ltd (Sillingy, France). Nano ZnO was purchased from Aoge Biotechnology Co., Ltd (Shanghai, China). The morphology of particles were measured using scanning electron microscopy (TM 1000, Hitachi Science Systems, Ltd., Japan) and Zetasizer Nano ZS (Malvern Instruments, Malvern, UK), and the data was showed in Supporting Information. The basal diet was used as negative control (NC) diet. The basal diet supplemented with standard commercial feed-grade ZnO (powder) at the dose of 3000 mg/kg (containing 2400 mg/kg Zn) was used as positive control (PC). Other two experimental diets were basal diet supplemented with 500 mg/kg porous ZnO (HiZ, containing 400 mg/kg Zn) and 500 mg/kg nano ZnO (ZNP, containing 400 mg/kg Zn), separately. All the diets were formulated to be iso-energetic and iso-nitrogenous and to meet the NRC (2012) nutrient requirements. All of the ZnO were pre-added in the Premix to allow they were at certain concentrations in different diets. The nutrient composition of basal diet is shown in Table 1. Piglets were allowed to have a 5-d adaption period before the trial begins. During the experimental period, piglets had free access to feed and drinking water at all times. Feed consumption from each pen was determined daily throughout the experimental period. Initial (day 0) and final (day 28) body weights were measured after 12 h fasting. Response variables measured included average daily feed intake (ADFI), average daily gain (ADG) and feed/gain ratio. The status of anal soft fecal contamination and swelling in each pen was examined and recorded every morning and afternoon during the experimental period. These data were then used to calculate the incidence rate of diarrhea according to the following formula:
Table 1. Composition of basal diet (as-fed basis)1.
| Ingredient | Content, % | Nutrient level | Content, % |
|---|---|---|---|
| Corn | 58.00 | DE (MJ/kg) | 14.01 |
| Soybean meal | 17.00 | CP | 22.84 |
| Extruded soybean | 7.00 | Ca | 0.86 |
| Fish meal | 4.50 | TP | 0.75 |
| Whey powder | 4.50 | Lys | 1.13 |
| Wheat bran | 5.00 | ||
| Dicalcium phosphate | 1.50 | ||
| Salt | 0.25 | ||
| Limestone | 1.10 | ||
| Lys | 0.15 | ||
| Premix2 | 1 | ||
| Total | 100 |
1The dietary treatments were: negative control (NC), basal diet; positive control (PC), basal diet + 3000ppm ZnO; low HiZox (LHZ), basal diet + 200 ppm HiZox; high HiZox (HHZ), basal diet + 500 ppm HiZox; ZnO nanoparticles (ZNP), basal diet + 500 ppm ZnO nanoparticles
2Premix supplied per kilogram of diet: VA1, 500 IU; VD3, 200 IU; VE, 10 IU; VK3, 0.5 mg; VB2, 3.6 mg; VB1, 1.0 mg; VB6, 1.5 mg; biotin, 0.05 mg; folic acid, 0.3 mg; d-pantothen, 10 mg; nicotinic acid, 10 mg; choline, 500 mg.
Sample collection
On day 28, 32 piglets (one piglet per pen) were randomly selected, and then killed according to our previous report [26]. Blood were sampled from a jugular vein, followed by the centrifugation at 3000g for 10 min at 4°C and stored at -80°C until analysis. Segments of longissimus dorsi muscle, liver, duodenum, jejunum and ileum were taken quickly. One part of the gut samples was kept in 10% neutral buffered formalin for histomorphometry analysis, other tissue samples were immediately frozen in liquid nitrogen and stored at -80°C for subsequent analysis.
Analysis of small intestinal morphology
The intestinal segments (20 mm) were embedded in paraffin. Sections (5 μm) were cut and stained. The stained sections were subsequently used to determine villus height (μm) and crypt depth (μm) according to previous study [27].
Analysis of blood samples
The serum concentrations of malondialdeyhde (MDA) and enzyme activities of diamine oxidase (DAO) and glutathione peroxidase (GSH-Px) were measured using spectrophotometric kits in accordance with the manufacturer’s instructions (Nanjing Jiangcheng Biotechnology Institute, China).
Analysis of trace metal elements in muscle and kidney
Tissue samples (5 g) were sliced and added into a mixture of perchloric acid and nitric acid (15mL, v:v = 1:4) overnight. Then the mixture were heated to 80°C for 2h, followed by 110°C for 1h, 150°C for 1h, and maintained at 220°C until dried. The ashed samples were suspended with 15 ml 1% nitric acid and filtered before analysis. The filtrated solution was then aspirated into inductively coupled plasma-optical emission spectroscopy (ICP-OES, Agilent, 720 ES). The concentrations of copper (Cu), zinc (Zn), ion (Fe), calcium (Ca), manganese (Mn), chromium (Cr) and magnesium (Mg) in muscle and kidney were determined.
RNA extraction and gene expression analysis
Total mRNA from duodenum, jejunum and ileum were isolated using TRIzol Reagent (TaKaRa, Dalian, China) according to the manufacturer’s instruction. The reverse transcription was performed according to our previous study [26]. Primers for interleukin 1 and 6 (IL-1, IL-6), tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), trefoil factor 3 (TFF3) and nuclear factor erythroid 2-related factor 2 (Nrf2) were list in Table 2. β-actin was used as a housekeeping gene to normalize the relative change of each mRNA. Real-time PCR was performed according to our previous studies [26].
Table 2. The primer sequences used in this study.
| Gene1 | Primer squence (5’-3’) | Size (bp) | References |
|---|---|---|---|
| IL-1 | F: GCTAACTACGGTGACAACAA R: TCTTCATCGGCTTCTCCACT |
196 | [28] |
| IL-6 | F: CCTGTCCACTGGGCACATAAC R: CAAGAAACAACCTGGCTCTGAAAC |
253 | [29] |
| TNF-α | F: CATCGCCGTCTCCTACCA R: CCCAGATTCAGCAAAGTCCA |
199 | [30] |
| IFN-γ | F: GAGCCAAATTGTCTCCTTCTAC R: CGAAGTCATTCAGTTTCCCAG |
140 | [31] |
| TFF3 | F: AGTGTGCCGTCCCTGCCAAG R: GCAGCCCCGGTTGTTGCACT |
80 | [32] |
| Nrf2 | F: GAAAGCCCAGTCTTCATTGC R: TTGGAACCGTGCTAGTCTCA |
190 | [33] |
| β-actin | F: CCAGGTCATCACCATCGG R: CCGTGTTGGCGTAGAGGT |
158 | [31] |
1 IL-1, interleukin 1; IL-6, interleukin 6; TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; TFF3, trefoil factor 3; Nrf2, nuclear factor erythroid 2-related factor 2.
Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) to test homogeneity of variances via Levene’s test and followed with Ducan’s multiple comparison test (SPSS18.0 software). Data is showed as the mean ± standard error of the mean. Values in the same row with different superscript are significant (P < 0.05), while values with the same superscript are not significantly different (P > 0.05).
Results
Growth performance and the incidence rate of diarrhea
The result of growth performance and the incidence rate of diarrhea were summarized in Table 3. From weanling to 28d post-weaning, piglets in PC and ZNP groups showed significantly higher ADG than NC group (P < 0.05). Piglets in HiZ group had similar diarrhea incidence compared with those in PC group (P > 0.05). Besides, HiZ group had less diarrhea compared with ZNP group (P > 0.05).
Table 3. Growth performance and the incidence rate of diarrhea.
| Item1 | NC | PC | HiZ | ZNP |
|---|---|---|---|---|
| ADG (g/d) | 329.91±23.34c | 420.09±7.57a | 367.41±13.38bc | 377.04±12.09ab |
| ADFI (g/d) | 601.82±11.17c | 699.93±23.89a | 651.61±9.59b | 675.77±5.57ab |
| F/G | 1.91±0.08a | 1.69±0.03b | 1.80±0.05ab | 1.87±0.02a |
| Diarrhea incidence | 9.15±0.08a | 4.91±0.10c | 5.13±0.07c | 5.51±0.10b |
1ADFI, average daily feed intake; ADG, average daily gain; F/G, feed/gain ratio. Data were shown as the mean ± SEM, n = 8.
abc Mean values within different letters were significantly different (P<0.05).
Trace metal elements in muscle and kidney
Dietary supplementation with different forms of ZnO had little impact on the concentrations of trace metal elements in muscle (Table 4). Piglets fed with or without ZnO did not influence the concentrations of trace metal elements in kidney, except for Zn. Piglets in PC group had the highest Zn concentration in kidney, while other three groups had similar Zn level in kidney.
Table 4. Concentrations of trace metal elements in muscle and kidney1.
| Organ | Metal | NC | PC | HiZ | ZNP |
|---|---|---|---|---|---|
| Muscle (μg/g) | Cr | 0.13±0.01 | 0.11±0.00 | 0.11±0.01 | 0.13±0.02 |
| Mn | 0.08±0.01 | 0.06±0.00 | 0.06±0.00 | 0.07±0.01 | |
| Cu | 0.31±0.01 | 0.27±0.02 | 0.29±0.01 | 0.29±0.02 | |
| Zn | 1.5±0.06 | 1.51±0.05 | 1.38±0.13 | 1.39±0.09 | |
| Fe | 1.62±0.08 | 1.18±0.04 | 1.23±0.10 | 1.33±0.12 | |
| Ca | 22.59±1.9 | 23.9±0.5 | 21.53±1.6 | 22.82±0.10 | |
| Mg | 24.67±0.59 | 24.58±0.87 | 21.76±2.00 | 23.5±0.10 | |
| Kidney (μg/g) | Cr | 0.09±0.01 | 0.09±0.00 | 0.1±0.01 | 0.1±0.01 |
| Mn | 0.29±0.02 | 0.26±0.01 | 0.28±0.01 | 0.27±0.01 | |
| Cu | 4.82±0.46 | 6.12±0.53 | 4.92±0.76 | 4.65±0.48 | |
| Zn | 8.17±1.06a | 20.23±1.38b | 5.85±0.42a | 6.71±0.62a | |
| Fe | 0.29±0.02 | 0.26±0.01 | 0.28±0.01 | 0.27±0.01 | |
| Ca | 21.84±0.61 | 25.54±2.48 | 19.69±1.07 | 21.32±2.09 | |
| Mg | 16.55±0.68 | 18.21±0.22 | 16.58±0.45 | 16.24±0.56 |
1 Data were shown as the mean ± SEM, n = 8
ab Mean values within different letters were significantly different (P<0.05).
DAO, GSH-Px and MDA in serum
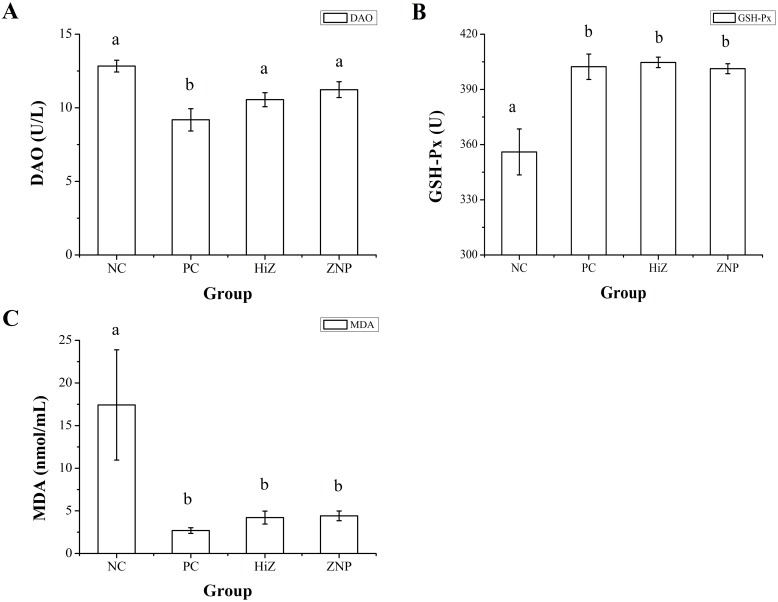
MDA concentration and activities of DAO and GSH-Px in serum were presented in Fig 1. Serum DAO activities were significant higher in NC group than in other groups (P < 0.05). Compared with NC group, piglets fed with ZnO (PC, HiZ and ZNP groups) had higher GSH-Px activities and lower MDA concentration in serum (P < 0.05). Piglets fed with HiZ and ZNP diets had no significant differences in DAO, GSH-Px and MDA levels (P > 0.05).
Fig 1. Activities of diamine oxidase (DAO) (A) and glutathione peroxidase (GSH-Px) (B), serum malondialdeyhde (MDA) concentration (C) in four groups.
Data were shown as the mean ± SEM, n = 8. a,bMean values within different letters were significantly different (P<0.05).
Intestinal morphology
As Table 5 showed that piglets in PC group had higher duodenal villus height and deeper jejunal crypt depth compared with other groups (P < 0.05). Piglets in PC, HiZ and ZNP group had deeper ileal crypt depth of than those in NC and LHZ group (P < 0.05).
Table 5. Effect of different sources of ZnO on intestinal morphology of weanling pigs1.
| Item (μm) | NC | PC | HiZ | ZNP | |
|---|---|---|---|---|---|
| Duodenum | Villus height | 386.60±8.73b | 408.32±5.95a | 376.73±2.42b | 373.63±9.15b |
| Crypt depth | 154.49±10.60 | 156.30±6.45 | 153.68±8.46 | 151.05±10.47 | |
| V/C2 | 2.50±0.18 | 2.61±0.15 | 2.49±0.14 | 2.41±0.15 | |
| Jejunum | Villus height | 334.73±11.27 | 360.58±13.66 | 355.79±26.18 | 352.89±13.81 |
| Crypt depth | 133.79±6.93b | 156.7±3.91a | 150.03±5.08ab | 133.79±7.95b | |
| V/C | 2.46±0.15 | 2.48±0.07 | 2.42±0.15 | 2.68±0.14 | |
| Ileum | Villus height | 320.40±15.01 | 305.40±20.21 | 321.03±16.78 | 322.45±14.86 |
| Crypt depth | 141.51±6.14a | 120.47±1.66b | 126.96±5.43b | 119.73±4.07b | |
| V/C | 2.30±0.11 | 2.59±0.16 | 2.43±0.13 | 2.50±0.20 |
1 Data were shown as the mean ± SEM, n = 8
2 V/C = Villus height/ Crypt depth
a,b Mean values within different letters were significantly different (P<0.05).
Intestinal inflammation
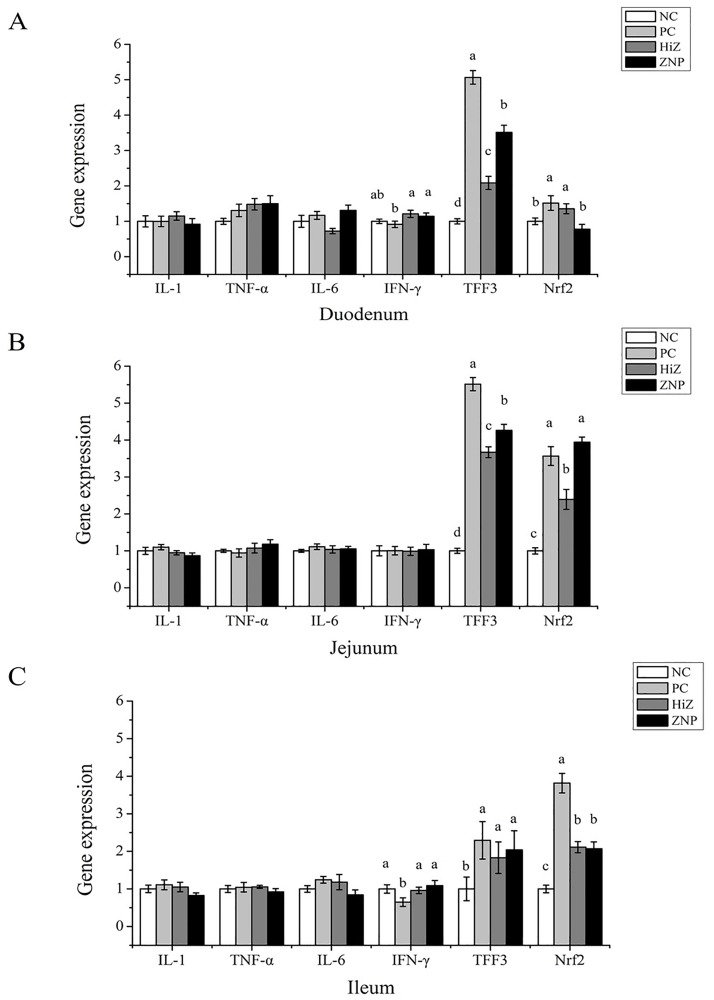
The result presented in Fig 2 showed that the dosage form of ZnO did not influence the mRNA expressions of IL-1, TNF-α and IL-6 in small intestine. PC diet down-regulated the mRNA level of IFN-γ in duodenum and ileum compared with HiZ and ZNP group (P < 0.05). Dietary supplementation with ZnO (PC, HiZ and ZNP group) up-regulated the mRNA levels of TFF3 and Nrf2 compared with NC group (P < 0.05). On the other side, in duodenum and ileum, the mRNA level of TFF3 is lower in HiZ group compared with ZNP group (P < 0.05). The duodenal mRNA level of Nrf2 is lower, while jejunal mRNA level of Nrf2 is higher in HiZ group compared with those in ZNP group (P < 0.05).
Fig 2. Intestinal relative mRNA levels of inflammatory cytokines.
IL-1, interleukin 1; IL-6, interleukin 6; TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; TFF3, trefoil factor 3; Nrf2, nuclear factor erythroid 2-related factor 2. Data were shown as the mean ± SEM, n = 8. a,bMean values within different letters were significantly different (P<0.05).
Discussion
Zn is an important micronutrient for the overall health and development of infants and young children. It has multiple effects on pathophysiological processes, including the absorptive and secretory processes [34, 35], the gut associated immune process [36], and the metabolic activity of the intestinal microbiota [37, 38]. Besides, numerous animal and human studies demonstrated the benefits of Zn supplementation on inflammation and infectious diseases [39, 40]. Therefore, Zn and Zn compounds have been frequently utilized as antimicrobial agents and growth promoter. In pig production, dietary supplementation with high doses of ZnO (2000–3000 mg/kg) have been frequently used to improve performance and to reduce the infectious diarrhea in newly-weaned piglets because of its antibacterial activity [41]. The antibacterial activity of ZnO is considered to be due to the generation of hydrogen peroxide (H2O2) from its surface [42]. Therefore, it is assumed that increasing the surface area of ZnO particle (decreasing particle size) can elevate the efficient of the H2O2 generation and the antibacterial activity. Osamu Yamamoto confirmed the suspect and found that the antibacterial activity of ZnO increased with decreasing particle size and increasing powder concentration [43]. Here we used low levels of porosity particles and nanoparticles of ZnO (both of them possess large surface layer) to investigate their potential to replace high dose of ZnO in piglet diets. We found that both porous and nano ZnO (HiZ and ZNP group) improved the growth performance, and showed similar or even better effect on reducing diarrhea than high-dose of regular ZnO (PC group). Besides, porous ZnO (HiZ group) had better effect on reducing diarrhea than nano ZnO (ZNP group). These results suggested that porous and nano ZnO possess more effective surface area than regular ZnO did under the same concentration. It also implied that further reducing the dose of porous and nano ZnO maybe still in effect.
To exert anti-diarrhea effect, the dosage of dietary regular ZnO needs to be high enough (approximately 2000–3000 mg/kg in diet of weaning piglet). Even when porous and nano ZnO are used as substitute, the dosage of ZnO added in feed is still higher than that of other Zn supplements, such as ZnSO4 and amino acid-chelated Zn. On the other side, the bioavailability of dietary ZnO is relative lower than ZnSO4 and amino acid-chelated Zn. Even so, there would be still a large amount of Zn enter the body by absorption through intestine when piglets fed with high level (pharmacological level) of ZnO [20]. Once Zn is absorbed by intestine, it is predominantly bound to proteins in the circulation [44]. Under normal circumstances, urinary loss of Zn is very low, and large amount of reabsorbed Zn would be stored in kidney waiting for transportation [45]. Here we found that feeding with regular ZnO at 3000 mg/kg (PC group) results in a large accumulation of Zn in kidney, which is extremely higher than other groups. However, high Zn in kidney may contribute to kidney stone formation, a common urinary condition that can cause excruciating pain [46]. Therefore, replacing high-dose dietary ZnO with low-dose porous and nano ZnO, in order to reduce Zn accumulation in kidney, is beneficial to the health of urinary system.
Zn is an important anti-stress factor. It is a fundamental element of more than 200 metalloenzymes, including many antioxidant enzymes, and affects activity and stability of many of them [47]. On the other side, weaning stress is often associated with oxidative stress and presented as the lipid peroxidation, elevated generation of MDA and reduced the activity of antioxidant enzymes [26]. And Zn plays an antioxidative role during weaning period in piglets. Thus, it is important to investigate the activities of antioxidant enzymes in weaning piglets when the usage of Zn is reduced. In this study, we found that dietary ZnO (PC, HiZ and ZNP group) significantly decreased the MDA level in serum compared with NC group, suggesting that both high dose of regular ZnO and low dose of porous and nano ZnO are able to reduce lipid peroxidation effectively. On the other side, ZnO has been reported to affect expression of proteins related to glutathione metabolism and favorably increased the expression of antioxidative proteins [48]. Elevation of GSH-Px activities may be an adaptive mechanism secondary to the increase of oxidative stress [49]. Consistent with this notion, we found that GSH-Px activity was significantly increased in PC, HiZ and ZNP group, implying that dietary supplementation with porous and nano ZnO at low concentration can effectively promote adaption to the oxidative stress as high level of regular ZnO did. DAO is an intracellular enzyme and widely distributed in intestinal villous of mammalians. Its activity is especially high in the jejunum and ileum [50]. An increase amount of DAO released into blood is considered as a signal of damage in intestinal mucosal integrity [51, 52]. Considering that Zn plays an important role in maintain epithelial barrier integrity and function [8, 53], we tested DAO activity in serum and found that piglets fed with basal diet (NC group) showed a higher DAO activity than other three groups, suggesting that low level of porous and nano ZnO, as well as high level of regular ZnO are beneficial in maintaining intestinal mucosal integrity. To get a better insight into the effect of different dosage forms of ZnO on the intestinal structure, we determined the intestinal villus height and crypt depth in piglets. A decrease in ileal crypt depth was observed in PC, HiZ and ZNP group, suggesting an effective protection against intestinal injury [27].
The weaning process is associated not only with intestinal integrity, but also with the intestinal inflammation. It has been well documented that weaning triggers the up-regulation of pro-inflammatory cytokines in the intestine, such as TNF-α, IL-6, IL-1β and INF-γ [54–56]. The inflammatory response and overproduction of pro-inflammatory cytokines result intestinal barrier dysfunction [54, 57]. In some studies, mRNA levels of TNF-α, IL-6 and IL-1 decreased with increasing concentration of dietary Zn in pigs [58, 59]. However, in the present study, dietary addition of ZnO (PC, HiZ and ZNP group) did not affect the mRNA expressions of IL-1, TNF-α and IL-6 after a 28-d feeding process. It has been demonstrated that weaning is associated with a transient up-regulation of inflammatory cytokine mRNA content on days 3 to 4 post weaning, and most of them rapidly return to pre-weaning values after day 9 post weaning [56, 58]. This might be why mRNA levels of IL-1, TNF-α and IL-6 were not affected by the dietary treatment in the present study. TFF3 and Nrf2 are important inflammatory cytokines. They have a major impact on maintenance of healthy mucosal surfaces [60, 61]. In the intestine, weaning could result in an increase of TFF3 mRNA levels, which is thought to be beneficial for the epithelial repair [62]. And increase of Nrf2 expression improves the expression of antioxidant genes and inhibits the expression of pro-inflammatory cytokines [63]. In this study, TFF3 and Nrf2 were up-regulated in the ZnO-fed group. Interestingly, ZNP supplementation had better effects on up-regulating TFF3 and Nrf2 levels in duodenum and jejunum than HiZ did, implying that nano ZnO may possess higher anti-inflammatory capacity than porous ZnO.
Conclusions
Dietary supplementation with low dose of porous and nano ZnO has similar (even better) effect on improving growth performance and intestinal morphology, reducing diarrhea and intestinal inflammatory as high dose of regular ZnO in weaning piglets. Compared with nano ZnO, porous ZnO has better effect on reducing diarrhea. But nano ZnO shows better effect on un-regulation of intestinal TFF3 and Nrf2 levels, suggesting it may possess higher anti-inflammatory capacity than porous ZnO. Overall, both porous and nano ZnO can be used as alternatives to high dose of regular ZnO in weaning piglets.
Supporting information
S1 Fig shows the morphology of the porous ZnO and nano ZnO through TEM. S1 Fig A shows that porous ZnO have a rough surface and exhibit a spongelike structure. S1 Fig B shows that nano ZnO are spherical in shape with a uniform size, and are found as aggregated particles.
(TIF)
S2 Fig A shows that approximately 80% of the porous ZnO particles have a size ranging from 0.1 mm to 0.2 mm. S2Fig B shows that nano ZnO have much smaller particle size (most of them are less than 0.1 μm) than porous ZnO.
(TIF)
Acknowledgments
We thank Animine Co., Ltd for providing porous ZnO (HiZox) in this study. We also thank staffs and postgraduate students of the center of healthy animal husbandry for collecting samples and technicians from key laboratory of agro-ecological processes in subtropical region for providing technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present work was supported by grants from the National Natural Science Foundation of China (No. 31501965) and National Key Research and Development Program of China (2016YFD0501201). Author Yonggang Zhang is employed by Animine Co., Ltd. Animine Co., Ltd provided porous ZnO (HiZox) for this study and provided support in the form of salary for author YZ, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the 'author contributions' section.
References
- 1.Kolodziejczakradzimska A, Jesionowski T. Zinc Oxide—From Synthesis to Application: A Review. Materials. 2014;7(4):2833–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozgur U, Alivov YI, Liu C, Teke A, Reshchikov MA, Dogan S, et al. A comprehensive review of ZnO materials and devices. Journal of Applied Physics. 2005;98(4):041301. [Google Scholar]
- 3.Wang Z, Burwinkel M, Chai W, Lange E, Blohm U, Breithaupt A, et al. Dietary Enterococcus faecium NCIMB 10415 and Zinc Oxide Stimulate Immune Reactions to Trivalent Influenza Vaccination in Pigs but Do Not Affect Virological Response upon Challenge Infection. PLoS ONE. 2014;9(1):e87007 10.1371/journal.pone.0087007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, Harrold RL, et al. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. Journal of Animal Science. 2001;79(4):934–41. 10.2527/2001.794934x [DOI] [PubMed] [Google Scholar]
- 5.Woll C. The chemistry and physics of zinc oxide surfaces. Progress in Surface Science. 2007;82(2):55–120. [Google Scholar]
- 6.Wahab R, Khan F, Singh R, Kaushik NK, Ahmad J, Siddiqui MA, et al. Utilization of photocatalytic ZnO nanoparticles for deactivation of safranine dye and their applications for statistical analysis. Physica E: Low-dimensional Systems and Nanostructures. 2015;69:101–8. [Google Scholar]
- 7.Liu H, Yang D, Yang H, Zhang H, Zhang W, Fang Y, et al. Comparative study of respiratory tract immune toxicity induced by three sterilisation nanoparticles: silver, zinc oxide and titanium dioxide. Journal of hazardous materials. 2013;248:478–86. 10.1016/j.jhazmat.2013.01.046 [DOI] [PubMed] [Google Scholar]
- 8.Hu CH, Song J, You Z, Luan ZS, Li W. Zinc Oxide–Montmorillonite Hybrid Influences Diarrhea, Intestinal Mucosal Integrity, and Digestive Enzyme Activity in Weaned Pigs. Biol Trace Elem Res. 2012;149(2):190–6. 10.1007/s12011-012-9422-9 [DOI] [PubMed] [Google Scholar]
- 9.Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Ågren MS. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair and Regeneration. 2007;15(1):2–16. 10.1111/j.1524-475X.2006.00179.x [DOI] [PubMed] [Google Scholar]
- 10.Wahab R, Siddiqui MA, Saquib Q, Dwivedi S, Ahmad J, Musarrat J, et al. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids and Surfaces B: Biointerfaces. 2014;117:267–76. 10.1016/j.colsurfb.2014.02.038 [DOI] [PubMed] [Google Scholar]
- 11.Wahab R, Kaushik N, Khan F, Kaushik NK, Choi EH, Musarrat J, et al. Self-Styled ZnO Nanostructures Promotes the Cancer Cell Damage and Supresses the Epithelial Phenotype of Glioblastoma. Scientific Reports. 2016;6:19950 10.1038/srep19950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra YK, Adelung R, Roehl C, Shukla D, Spors F, Tiwari V. Virostatic potential of micro–nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Research. 2011;92(2):305–12. 10.1016/j.antiviral.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madec F, Bridoux N, Bounaix S, Cariolet R, Duval-Iflah Y, Hampson DJ, et al. Experimental models of porcine post-weaning colibacillosis and their relationship to post-weaning diarrhoea and digestive disorders as encountered in the field. Veterinary Microbiology. 2000;72(3–4):295–310. 10.1016/S0378-1135(99)00202-3. [DOI] [PubMed] [Google Scholar]
- 14.Laine T, Lyytikainen T, Yliaho M, Anttila M. Risk factors for post-weaning diarrhoea on piglet producing farms in Finland. Acta Veterinaria Scandinavica. 2008;50(1):21-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulsen HD. Zinc oxide for weaned pigs. Rome, Italy: 4th Annu. Meet. Eur. Assoc. Anim. Prod. EAAP; 1989. p. 8–10 in Proc.
- 16.Ou D, Li D, Cao Y, Li X, Yin J, Qiao S, et al. Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs. Journal of Nutritional Biochemistry. 2007;18(12):820–6. 10.1016/j.jnutbio.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 17.Sales J. Effects of Pharmacological Concentrations of Dietary Zinc Oxide on Growth of Post-weaning Pigs: A Meta-analysis. Biol Trace Elem Res. 2013;152(3):343–9. 10.1007/s12011-013-9638-3 [DOI] [PubMed] [Google Scholar]
- 18.Davis ME, Brown DC, Maxwell CV, Johnson ZB, Kegley EB, Dvorak R. Effect of phosphorylated mannans and pharmacological additions of zinc oxide on growth and immunocompetence of weanling pigs. Journal of Animal Science. 2004;82(2):581–7. [DOI] [PubMed] [Google Scholar]
- 19.Bednorz C, Oelgeschläger K, Kinnemann B, Hartmann S, Neumann K, Pieper R, et al. The broader context of antibiotic resistance: Zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. International Journal of Medical Microbiology. 2013;303(6–7):396–403. 10.1016/j.ijmm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Schell TC, Kornegay ET. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-methionine, Zn-lysine, or ZnSO4. Journal of Animal Science. 1996;74(7):1584–93. 10.2527/1996.7471584x [DOI] [PubMed] [Google Scholar]
- 21.Wei B, Yang L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchemical Journal. 2010;94(2):99–107. [Google Scholar]
- 22.Jang I, Kwon CH, Ha DM, Jung DY, Kang SY, Park MJ, et al. Effects of a lipid-encapsulated zinc oxide supplement on growth performance and intestinal morphology and digestive enzyme activities in weanling pigs. Journal of Animal Science and Technology. 2014;56(1):29-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morales J, Cordero G, Pineiro C, Durosoy S. Zinc oxide at low supplementation level improves productive performance and health status of piglets. Journal of Animal Science. 2012;90:436–8. 10.2527/jas.53833 [DOI] [PubMed] [Google Scholar]
- 24.Song ZH, Ke YL, Xiao K, Jiao LF, Hong QH, Hu CH. Diosmectite-zinc oxide composite improves intestinal barrier restoration and modulates TGF-β1, ERK1/2, and Akt in piglets after acetic acid challenge. Journal of Animal Science. 2015;93(4):1599–607. 10.2527/jas.2014-8580 [DOI] [PubMed] [Google Scholar]
- 25.Cho W, Kang B, Lee JK, Jeong J, Che J, Seok S. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Particle and Fibre Toxicology. 2013;10(1):9-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin J, Liu M, Ren W, Duan J, Yang G, Zhao Y, et al. Effects of Dietary Supplementation with Glutamate and Aspartate on Diquat-Induced Oxidative Stress in Piglets. PLoS ONE. 2015;10(4):e0122893 10.1371/journal.pone.0122893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan Z, Liu S, Zhou Y, Mi S, Liu G, Wu X, et al. Chlorogenic Acid Decreases Intestinal Permeability and Increases Expression of Intestinal Tight Junction Proteins in Weaned Rats Challenged with LPS. PLoS ONE. 2014;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie C, Wu X, Long C, Wang Q, Fan Z, Li S, et al. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Veterinary Research. 2016;12(1):243 10.1186/s12917-016-0872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YL, Shi JX, Lu J, Che ZQ, Zhu HL, Hou YQ, et al. Up-regulated expression of peroxisome proliferator-activated receptor γ in the hypothalamic–pituitary–adrenal axis of weaned pigs after Escherichia coli lipopolysaccharide challenge. The Veterinary Journal. 2010;184(2):230–5. 10.1016/j.tvjl.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhou P, Zhai S, Zhou X, Lin P, Jiang T, Hu X, et al. Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo. Int J Biol Sci. 2011;7(7):947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou Y, Xiang Q, Wang J, Peng J, Wei H. Oregano Essential Oil Improves Intestinal Morphology and Expression of Tight Junction Proteins Associated with Modulation of Selected Intestinal Bacteria and Immune Status in a Pig Model. BioMed Research International. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P, Pieper R, Tedin L, Martin L, Meyer W, Rieger J, et al. Effect of dietary zinc oxide on jejunal morphological and immunological characteristics in weaned piglets. Journal of Animal Science. 2014;92(11):5009–18. 10.2527/jas.2013-6690 [DOI] [PubMed] [Google Scholar]
- 33.Zou Y, Wang J, Peng J, Wei H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxidative Medicine and Cellular Longevity. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson D, Sehested J, Feng Z, Poulsen HD. Zinc is involved in regulation of secretion from intestinal epithelium in weaned piglets. Livestock Science. 2007;108(1):45–8. [Google Scholar]
- 35.Carlson D, Poulsen HD, Sehested J. Influence of weaning and effect of post weaning dietary zinc and copper on electrophysiological response to glucose, theophylline and 5-HT in piglet small intestinal mucosa. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2004;137(4):757–65. [DOI] [PubMed] [Google Scholar]
- 36.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. The American Journal of Clinical Nutrition. 1998;68(2):447S–63S. [DOI] [PubMed] [Google Scholar]
- 37.Starke IC, Zentek J, Vahjen W. Ex vivo-growth response of porcine small intestinal bacterial communities to pharmacological doses of dietary zinc oxide. PLoS ONE. 2013;8(2):e56405 10.1371/journal.pone.0056405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahjen W, Pieper R, Zentek J. Bar-coded pyrosequencing of 16S rRNA gene amplicons reveals changes in ileal porcine bacterial communities due to high dietary zinc intake. Applied and Environmental Microbiology. 2010;76(19):6689–91. 10.1128/AEM.03075-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulk C, Burnett D, Tokach M, Nelssen J, Dritz SS, DeRouchey J, et al. Effect of added zinc in diets with ractopamine hydrochloride on growth performance, carcass characteristics, and ileal mucosal inflammation mRNA expression of finishing pigs. Journal of Animal Science. 2015;93(1):185–96. 10.2527/jas.2014-8286 [DOI] [PubMed] [Google Scholar]
- 40.Wei Z, Burwinkel M, Palissa C, Ephraim E, Schmidt MFG. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Veterinary Microbiology. 2012;160(3):468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulsen HD. Zinc oxide for weanling piglets. Acta Agriculturae Scandinavica A-Animal Sciences. 1995;45(3):159–67. [Google Scholar]
- 42.Sawai J, Shoji S, Igarashi H, Hashimoto A, Kokugan T, Shimizu M, et al. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. Journal of Fermentation and Bioengineering. 1998;86(5):521–2. 10.1016/S0922-338X(98)80165-7. [DOI] [Google Scholar]
- 43.Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. International Journal of Inorganic Materials. 2001;3(7):643–6. 10.1016/S1466-6049(01)00197-0. [DOI] [Google Scholar]
- 44.Paterson PG, Mas A, Sarkar B, Zlotkin SH. The Influence of Zinc-Binding Ligands in Fetal Circulation on Zinc Clearance Across the In Situ Perfused Guinea Pig Placenta. Journal of Nutrition. 1991;121(3):338–44. [DOI] [PubMed] [Google Scholar]
- 45.Victery W, Smith JM, Vander AJ. Renal tubular handling of zinc in the dog. American Journal of Physiology-Renal Physiology. 1981;241(5):F532–F9. [DOI] [PubMed] [Google Scholar]
- 46.Chi T, Kim MS, Lang S, Bose N, Kahn A, Flechner L, et al. A Drosophila Model Identifies a Critical Role for Zinc in Mineralization for Kidney Stone Disease. PLoS ONE. 2015;10(5):e0124150 10.1371/journal.pone.0124150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Advances in Nutrition: An International Review Journal. 2013;4(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Ou D, Yin J, Wu G, Wang J. Proteomic analysis reveals altered expression of proteins related to glutathione metabolism and apoptosis in the small intestine of zinc oxide-supplemented piglets. Amino Acids. 2009;37(1):209–18. 10.1007/s00726-009-0242-y [DOI] [PubMed] [Google Scholar]
- 49.Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Research. 2006;1090(1):35–44. 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang C, Sun H, Wu J, Niu HH, Feng J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. Journal of Animal Physiology and Animal Nutrition. 2014;98(4):680–5. 10.1111/jpn.12122 [DOI] [PubMed] [Google Scholar]
- 51.Wolvekamp MCJ, De Bruin RWF. Diamine Oxidase: An Overview of Historical, Biochemical and Functional Aspects. Digestive Diseases. 1994;12(1):2–14. [DOI] [PubMed] [Google Scholar]
- 52.Hu CH, Gu LY, Luan ZS, Song J, Zhu K. Effects of montmorillonite–zinc oxide hybrid on performance, diarrhea, intestinal permeability and morphology of weanling pigs. Animal Feed Science and Technology. 2012;177(1):108–15. [Google Scholar]
- 53.Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E. Zinc Oxide Protects Cultured Enterocytes from the Damage Induced by Escherichia coli. Journal of Nutrition. 2003;133(12):4077–82. [DOI] [PubMed] [Google Scholar]
- 54.Hu CH, Xiao K, Luan ZS, Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. Journal of Animal Science. 2013;91(3):1094–101. 10.2527/jas.2012-5796 [DOI] [PubMed] [Google Scholar]
- 55.Mccracken BA, Spurlock ME, Roos M, Zuckermann FA, Gaskins HR. Weaning Anorexia May Contribute to Local Inflammation in the Piglet Small Intestine. Journal of Nutrition. 1999;129(3):613–9. [DOI] [PubMed] [Google Scholar]
- 56.Pie S, Lalles JP, Blazy F, Laffitte J, Seve B, Oswald IP. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. Journal of Nutrition. 2004;134(3):641–7. [DOI] [PubMed] [Google Scholar]
- 57.Kim JC, Hansen CF, Mullan BP, Pluske JR. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Animal Feed Science and Technology. 2012;173(1):3–16. [Google Scholar]
- 58.Hu C, Song J, Li Y, Luan Z, Zhu K. Diosmectite–zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. British Journal of Nutrition. 2013;110(04):681–8. [DOI] [PubMed] [Google Scholar]
- 59.Hu CH, Xiao K, Song J, Luan ZS. Effects of zinc oxide supported on zeolite on growth performance, intestinal microflora and permeability, and cytokines expression of weaned pigs. Animal Feed Science and Technology. 2013;181(1):65–71. [Google Scholar]
- 60.Scholven J, Taras D, Sharbati S, Schon J, Gabler C, Huber O, et al. Intestinal Expression of TFF and Related Genes During Postnatal Development in a Piglet Probiotic Trial. Cellular Physiology and Biochemistry. 2009;23:143–56. 10.1159/000204103 [DOI] [PubMed] [Google Scholar]
- 61.Song ZH, Tong G, Xiao K, Jiao LF, Ke YL, Hu CH. L-Cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immunity. 2016;22(3):152–61. 10.1177/1753425916632303 [DOI] [PubMed] [Google Scholar]
- 62.Lin J, Holzman IR, Jiang P, Babyatsky MW. Expression of Intestinal Trefoil Factor in Developing Rat Intestine. Neonatology. 1999;76(2):92–7. [DOI] [PubMed] [Google Scholar]
- 63.Satsu H, Chidachi E, Hiura Y, Ogiwara H, Gondo Y, Shimizu M. Induction of NAD (P) H: quinone oxidoreductase 1 expression by cysteine via Nrf2 activation in human intestinal epithelial LS180 cells. Amino Acids. 2012;43(4):1547–55. 10.1007/s00726-012-1230-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Fig shows the morphology of the porous ZnO and nano ZnO through TEM. S1 Fig A shows that porous ZnO have a rough surface and exhibit a spongelike structure. S1 Fig B shows that nano ZnO are spherical in shape with a uniform size, and are found as aggregated particles.
(TIF)
S2 Fig A shows that approximately 80% of the porous ZnO particles have a size ranging from 0.1 mm to 0.2 mm. S2Fig B shows that nano ZnO have much smaller particle size (most of them are less than 0.1 μm) than porous ZnO.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.