Abstract
Rationale: Mechanisms contributing to chronic lung disease after preterm birth are incompletely understood.
Objectives: To identify antenatal risk factors associated with increased risk for bronchopulmonary dysplasia (BPD) and respiratory disease during early childhood after preterm birth, we performed a prospective, longitudinal study of 587 preterm infants with gestational age less than 34 weeks and birth weights between 500 and 1,250 g.
Methods: Data collected included perinatal information and assessments during the neonatal intensive care unit admission and longitudinal follow-up by questionnaire until 2 years of age.
Measurements and Main Results: After adjusting for covariates, we found that maternal smoking prior to preterm birth increased the odds of having an infant with BPD by twofold (P = 0.02). Maternal smoking was associated with prolonged mechanical ventilation and respiratory support during the neonatal intensive care unit admission. Preexisting hypertension was associated with a twofold (P = 0.04) increase in odds for BPD. Lower gestational age and birth weight z-scores were associated with BPD. Preterm infants who were exposed to maternal smoking had higher rates of late respiratory disease during childhood. Twenty-two percent of infants diagnosed with BPD and 34% of preterm infants without BPD had no clinical signs of late respiratory disease during early childhood.
Conclusions: We conclude that maternal smoking and hypertension increase the odds for developing BPD after preterm birth, and that maternal smoking is strongly associated with increased odds for late respiratory morbidities during early childhood. These findings suggest that in addition to the BPD diagnosis at 36 weeks, other factors modulate late respiratory outcomes during childhood. We speculate that measures to reduce maternal smoking not only will lower the risk for preterm birth but also will improve late respiratory morbidities after preterm birth.
Keywords: prematurity, maternal smoking, bronchopulmonary dysplasia, hypertensive disorders of pregnancy, preeclampsia
At a Glance Commentary
Scientific Knowledge on the Subject
Despite improvements in perinatal care, preterm infants remain at high risk for developing bronchopulmonary dysplasia (BPD) and late respiratory disease during childhood. Antenatal factors that contribute to the development of BPD and late respiratory problems during infancy are incompletely understood. In addition, the relationship between risk for BPD and late respiratory sequelae are uncertain.
What This Study Adds to the Field
Data derived from this prospective, longitudinal multicenter study show that maternal smoking and preexisting hypertension increase the odds for developing BPD after preterm birth and that maternal smoking is strongly associated with increased odds for late respiratory morbidities during early childhood. These findings suggest that in addition to BPD diagnosis at 36 weeks corrected age, other factors modulate late respiratory outcomes during childhood.
Despite improvements in perinatal care, preterm children remain at high risk for mortality and significant respiratory morbidities owing to the development of bronchopulmonary dysplasia (BPD) (1). BPD is the chronic lung disease of prematurity that develops in infants who require respiratory support at birth owing to immaturity of the preterm lung (2). BPD occurs in roughly 45% of infants born at less than or equal to 29 weeks of gestation with birth weights between 400 and 1,500 g, with approximately 10,000–15,000 new cases reported each year (1, 3, 4). The incidence of BPD has not changed over the past few decades, reflecting improved survival of extremely preterm infants who are at highest risk for BPD (5).
BPD is also associated with significant neonatal intensive care unit (NICU)-related complications, including the prolonged need for mechanical ventilation, respiratory support, and oxygen therapy; longer duration of hospitalization; and higher rates of nonrespiratory comorbidities, such as retinopathy of prematurity and brain injury (5, 6). After NICU discharge, infants with BPD often require frequent hospital readmissions and have high rates of emergency room or physician visits for recurrent respiratory exacerbations, infections, and reactive airway disease. Sustained abnormalities of lung function, poor exercise tolerance, and the need for chronic respiratory medications throughout childhood and adolescence are also increased in former preterm infants (7, 8). Past studies have shown that over 50% of preterm infants subsequently require rehospitalizations or chronic respiratory medications after NICU discharge, including preterm infants without a formal diagnosis of BPD (8). Controversies persist regarding how best to define BPD and whether bearing this diagnosis at 36 weeks postmenstrual age (PMA) adequately reflects the late risk for lung disease during childhood and into adult life (9–11).
Although postnatal factors, such as hyperoxia, mechanical ventilation, prolonged patency of the ductus arteriosus, sepsis, inflammation, and others, increase the risk for BPD, epidemiologic studies have further identified important roles for antenatal factors as well (12–19). Adverse antenatal factors, such as chorioamnionitis, preeclampsia, preexisting hypertensive disorders, obesity, and others, have been variably associated with an increased risk for BPD (11, 15–22). Maternal smoking has also been linked with an increased rate of preterm birth (23, 24). In addition, past studies further suggest that maternal smoking is associated with a higher risk for lung disease in children (25–27).
Recently, participants in an NHLBI-sponsored workshop discussed the importance of prenatal and early postnatal influences on lung growth and development on subsequent respiratory function and disease throughout childhood (11). This workshop further highlighted major gaps in understanding of how environmental and maternal factors can impact late respiratory outcomes during early childhood, and that the exact relationships between prenatal exposures and early postnatal events on the subsequent development of late respiratory disease during infancy, especially after preterm birth, remain uncertain. Because an increasing number of studies have shown that preterm birth alone is associated with late respiratory disease in childhood, links between the diagnosis of BPD at 36 weeks corrected age and persistent chronic lung disease during infancy and beyond remain unclear.
To better understand antenatal risk factors associated with the development of BPD and late respiratory disease during early childhood after premature birth, and to examine the relationship between the diagnosis of BPD and respiratory disease after NICU discharge, we completed a prospective longitudinal study of a cohort of preterm infants. We collected extensive perinatal data and performed serial assessments during NICU admission and longitudinal follow-up by questionnaire to evaluate the potential contributions of antenatal factors that increase the risk for BPD, modulate the clinical severity of BPD, and contribute to persistent respiratory disease throughout early infancy. We further examined the relationship between the current diagnosis of BPD at 36 weeks PMA and subsequent respiratory morbidities during early childhood.
Methods
Study Population
All data were obtained between July 2006 and November 2016 as part of a prospective observational research protocol that was reviewed and approved by the institutional review boards of the respective sites. Written informed consent was received from the parents or guardians of all participants. The study population consisted of subjects enrolled in a prospective study of premature infants at risk for BPD (NHLBI grant HL085703). Participants were preterm infants from five centers who had a gestational age less than or equal to 34 weeks, birth within the previous 7 days, and a birth weight between 500 and 1,250 g.
Study Design
Data were prospectively collected and managed using a REDCap database hosted at the University of Colorado Denver (28). BPD status and severity were assessed at 36 weeks PMA using a modification of the National Institutes of Health workshop definition (3) with application of the oxygen reduction test (29). For some analyses, comparisons were made regarding the presence or absence of BPD, which was defined as children having either no or mild BPD versus those diagnosed with moderate or severe BPD. Birth weight z-scores were calculated by the methods of Oken and colleagues (30). Maternal smokers were self-identified at the initial patient assessment. A newborn was considered as small for gestational age if the birth weight z-score was below the 10th percentile for sex and gestational age. For subjects with a small number of missing variables (three or fewer), their medical records were assumed to be complete, and the missing values were imputed, except for the following variables, which were not changed: corticosteroids, pregestational diabetes, antibiotic use, maternal fever, or tocolytic medications. Variables with missing values for all other records were left as unknown. The number of events restricted our number of predictors in the model to 20, which were selected on the basis of a priori clinical knowledge. We excluded four subjects with three or more missing variables from the analysis rather than attempting to impute a large proportion of missing information for a given subject. Follow-up surveys were administered at 6-month intervals to examine respiratory health over the first 2 years of life, which included questions regarding environmental exposures to secondhand smoke and to pets. A child was considered to have a diagnosis of late respiratory disease if any of the following events occurred over the first 2 years of life: one or more respiratory hospitalizations; use of inhaled steroids, inhaled bronchodilators, and/or diuretics; and a physician’s diagnosis of asthma, reactive airway disease, or a BPD exacerbation.
Statistical Analysis
Chi-square tests, Fisher’s exact tests, and Wilcoxon signed-rank tests were used to assess associations across BPD and late respiratory outcome status for categorical and continuous variables, respectively. A logistic regression model was fitted using an outcome of moderate or severe BPD at 36 weeks PMA. Perinatal risk factors and potential confounders were identified a priori on the basis of clinical importance and were restricted to an appropriate number based on the number of events (see Table 1) (31). To facilitate clinical interpretation, signs were reversed for birth weight z-score, maternal age, and gestational age. A similar logistic regression was fitted modeling the diagnosis of late respiratory disease using a reduced set of the covariates from the prior model. Interactions of maternal smoking with all other covariates were tested. Two logistic regressions were fitted on the diagnosis of late respiratory disease using dichotomized BPD and the classification of BPD severity as the only predictors. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Table 1.
Subject Characteristics by Bronchopulmonary Dysplasia Status
| All Subjects (n = 587) | No or Mild BPD (n = 345) | Moderate or Severe BPD (n = 242) | P Value* | |
|---|---|---|---|---|
| Birth weight, g | 930 (758 to 1,080) | 1,000 (855 to 1,125) | 800 (680 to 970) | <0.01 |
| Birth weight z-score | −0.24 (−0.82 to 0.29) | −0.24 (−0.94 to 0.29) | −0.25 (−0.69 to 0.29) | 0.20 |
| Birth weight strata, g | ||||
| 500–749 (n = 136) | 136 (23%) | 46 (13.3%) | 90 (37.2%) | <0.01 |
| 750–999 (n = 220) | 223 (37.7%) | 123 (35.7%) | 100 (41.3%) | 0.16 |
| 1,000–1,250 (n = 219) | 228 (38.6%) | 176 (51%) | 52 (21.5%) | <0.01 |
| Small for gestational age | 158 (26.7%) | 92 (26.7%) | 66 (27.3%) | 0.87 |
| Birth length, cm | 35 (32.5 to 37) | 35.5 (34 to 37.2) | 33 (31.5 to 35.6) | <0.01 |
| Head circumference, cm | 24.5 (23 to 26) | 25.05 (24 to 26.5) | 23.5 (22 to 25) | <0.01 |
| Gestational age, wk | 27 (26 to 28) | 27 (26 to 29) | 26 (24 to 27) | <0.01 |
| Maternal age, yr | 28 (23 to 32) | 28 (23 to 33) | 28 (23 to 31) | 0.14 |
| Male sex | 299 (50.6%) | 164 (47.5%) | 135 (55.8%) | 0.05 |
| Maternal race | ||||
| Asian | 7 (1.2%) | 4 (1.2%) | 3 (1.2%) | 0.93 |
| Black or African American | 119 (20.1%) | 77 (22.3%) | 42 (17.4%) | 0.14 |
| Hawaiian or Pacific Islander | 0 (0%) | 0 (0%) | 0 (0%) | — |
| White | 457 (77.3%) | 262 (75.9%) | 195 (80.6%) | 0.18 |
| Other | 1 (0.2%) | 0 (0%) | 1 (0.4%) | — |
| Unknown | 3 (0.5%) | 3 (0.9%) | 0 (0%) | 0.15 |
| Maternal ethnicity | ||||
| Hispanic or Latino | 119 (20.1%) | 73 (21.2%) | 46 (19.0%) | 0.52 |
| Not Hispanic or Latino | 467 (79.0%) | 271 (78.6%) | 196 (81.0%) | 0.47 |
| Multiple gestations | 143 (24.2%) | 87 (25.2%) | 56 (23.1%) | 0.56 |
| Antenatal corticosteroids | 487 (82.4%) | 289 (83.8%) | 198 (81.8%) | 0.42 |
| Cesarean section delivery | 408 (69.0%) | 250 (72.5%) | 158 (65.3%) | 0.06 |
| Intubated in delivery room | 363 (61.4%) | 180 (52.2%) | 183 (75.6%) | 0.02 |
| Intubated in NICU | 122 (20.6%) | 74 (21.4%) | 48 (19.8%) | 0.11 |
| Surfactant in delivery room | 460 (77.8%) | 239 (69.3%) | 221 (91.3%) | <0.01 |
| PDA medical treatment | 245 (41.5%) | 120 (34.8%) | 125 (51.7%) | <0.05 |
| PDA surgical ligation | 74 (12.5%) | 21 (6.1%) | 53 (21.9%) | <0.01 |
| IVH grade 3 or 4 | 24 (4.1%) | 8 (2.3%) | 16 (6.6%) | 0.01 |
| Threshold ROP | 47 (8.0%) | 8 (2.3%) | 39 (16.1%) | <0.01 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; IVH = intraventricular hemorrhage; NICU = neonatal intensive care unit; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity.
Descriptive statistics [count (percent) or median (interquartile range)] are displayed for the demographics of the study population, stratified by bronchopulmonary dysplasia severity. Univariate comparisons were made across bronchopulmonary dysplasia severity outcomes.
P value denotes comparison of last two columns.
Results
Characteristics of the 587 infants enrolled across sites are stratified by BPD status (Table 1). The median birth weight and gestational age were 930 g and 27 weeks, respectively. Most of the mothers in this study were non-Hispanic (79%) and white (77%). Smoking was reported by 14% of mothers (Table 2). The diagnosis of BPD disease severity included mild (n = 214 [36.5%]), moderate (n = 119 [20.3%]), and severe (n = 123 [21.0%]) categories. Applying a dichotomized diagnosis of BPD status, 242 (41.2%) infants developed moderate or severe BPD, and 345 (58.8%) infants did not develop BPD (n = 131 [22.3%]) or developed only mild disease (n = 214 [36.5%]). More infants with moderate and severe BPD had severe intraventricular hemorrhage (P = 0.01) and more often were treated with surgical ligation of a patent ductus arteriosus (P < 0.01) than those with no or mild BPD (Table 1).
Table 2.
Maternal Complications by Bronchopulmonary Dysplasia Status
| All Subjects (n = 587) | No or Mild BPD (n = 345) | Moderate or Severe BPD (n = 242) | P Value* | |
|---|---|---|---|---|
| Preexisting diabetes | 22 (3.7%) | 15 (4.3%) | 7 (2.9%) | 0.33 |
| Gestational diabetes | 40 (6.8%) | 27 (7.8%) | 13 (5.4%) | 0.24 |
| Preexisting hypertension | 67 (11.3%) | 37 (10.7%) | 30 (12.4%) | 0.55 |
| Prolonged rupture of membranes | 107 (18.1%) | 64 (18.6%) | 43 (17.8%) | 0.86 |
| Chorioamnionitis | 110 (18.6%) | 61 (17.7%) | 49 (20.2%) | 0.38 |
| Preeclampsia | 154 (26.1%) | 98 (28.4%) | 56 (23.1%) | 0.17 |
| Antepartum hemorrhage | 67 (11.3%) | 33 (9.6%) | 34 (14.0%) | 0.09 |
| Maternal smoking | 80 (13.5%) | 35 (10.1%) | 45 (18.6%) | <0.01 |
| Maternal alcohol use | 13 (2.2%) | 6 (1.7%) | 7 (2.9%) | 0.35 |
| Maternal substance abuse | 50 (8.5%) | 27 (7.8%) | 23 (9.5%) | 0.47 |
Definition of abbreviation: BPD = bronchopulmonary dysplasia.
Descriptive statistics [count (percent)] are presented for maternal complications calculated for the entire study population, stratified by bronchopulmonary dysplasia severity. Univariate comparisons were made across bronchopulmonary dysplasia severity outcomes.
P value denotes comparison of last two columns.
A univariate analysis between antenatal events and dichotomized BPD status yielded a significant relationship between maternal smoking and the development of BPD after preterm birth (P < 0.01) (Table 2). Of those children who developed moderate or severe BPD, 18.6% had mothers who smoked during pregnancy. Of those who developed mild or no BPD, 10.1% had mothers who smoked during pregnancy. None of the other antenatal events were significantly associated with BPD.
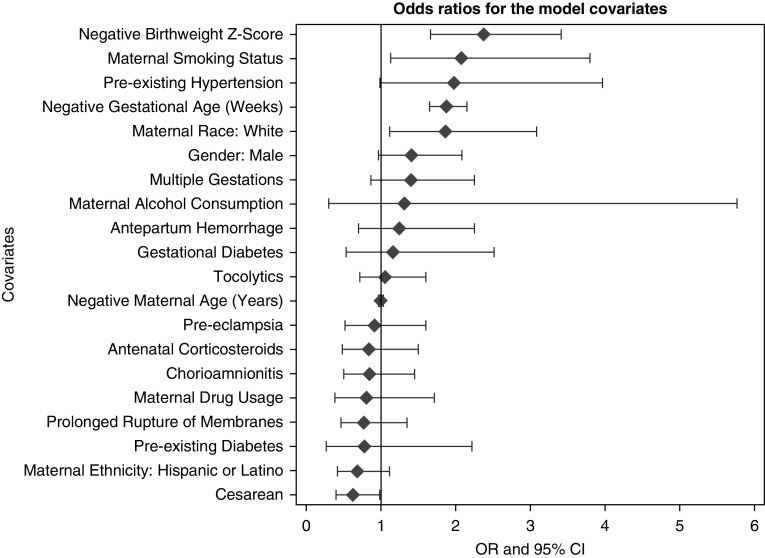
A multiple linear logistic regression was fitted to the diagnosis of BPD (Table 3) (c-index, 0.77). There was a significant association between maternal smoking and BPD severity. After adjusting for covariates, we found that mothers who smoked while pregnant increased their odds of having an infant with moderate or severe BPD by 2.02-fold (95% confidence interval (CI), 1.09–3.74; P = 0.02). Preexisting maternal hypertension was associated with a 2.11 times increase in the odds of BPD severity (95% CI, 1.05–4.24; P = 0.04). Children of white mothers had a 1.85-times increase in the odds of BPD severity at 36 weeks than those of other races (95% CI, 1.11–3.08; P = 0.02). As expected, lower gestational age (weeks) (odds ratio [OR], 1.89; 95% CI, 1.65–2.17; P < 0.01) and lower birth weight z-scores (OR, 2.40; 95% CI, 1.66–3.46; P < 0.01) were also significantly associated with an increase in BPD severity. Cesarean section delivery was associated with a lower odds (OR, 0.63; 95% CI, 0.40–0.99; P = 0.05). The ORs from the model are ranked graphically in Figure 1. Interactions of maternal smoking with all other covariates were tested, and none yielded significant associations with smoking status, including degree of prematurity.
Table 3.
Model Results of Logistic Regression for Bronchopulmonary Dysplasia Status
| Parameter | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Decreasing birth weight z-score, 1 SD | 2.40 | 1.66–3.46 | <0.01 |
| Male sex | 1.37 | 0.92–2.03 | 0.12 |
| Maternal race: white | 1.85 | 1.11–3.08 | 0.02 |
| Maternal ethnicity: Hispanic or Latino | 0.70 | 0.42–1.17 | 0.17 |
| Decreasing gestational age, wk | 1.89 | 1.65–2.17 | <0.01 |
| Maternal smoking status | 2.02 | 1.09–3.74 | 0.02 |
| Antenatal corticosteroids | 0.90 | 0.51–1.59 | 0.71 |
| Multiple gestations | 1.50 | 0.92–2.44 | 0.10 |
| Cesarean section delivery | 0.63 | 0.40–0.99 | 0.05 |
| Preexisting diabetes | 0.92 | 0.32–2.65 | 0.87 |
| Gestational diabetes | 1.23 | 0.56–2.67 | 0.61 |
| Preexisting hypertension | 2.11 | 1.05–4.24 | 0.04 |
| Prolonged rupture of membranes | 0.86 | 0.50–1.48 | 0.59 |
| Chorioamnionitis | 0.87 | 0.51–1.48 | 0.60 |
| Preeclampsia | 0.97 | 0.55–1.70 | 0.92 |
| Antepartum hemorrhage | 1.13 | 0.62–2.05 | 0.69 |
| Decreasing maternal age, yr | 1.01 | 0.98–1.05 | 0.57 |
| Maternal alcohol consumption | 0.97 | 0.20–4.70 | 0.97 |
| Maternal drug use | 0.94 | 0.44–2.02 | 0.88 |
| Tocolytics | 1.08 | 0.72–1.63 | 0.71 |
Risk factors for bronchopulmonary dysplasia: parametric estimates, Wald 95% confidence intervals, and P values derived from the logistic regression analysis modeling bronchopulmonary dysplasia severity.
Figure 1.
Odds ratios (ORs) with 95% confidence intervals (CIs) for model covariates from the logistic regression analysis modeling bronchopulmonary dysplasia severity.
To further investigate the impact of maternal smoking, univariate analyses between postnatal NICU events and maternal smoking status were performed (Table 4). The requirement for continuous positive airway pressure at 36 weeks PMA was marginally greater in infants from maternal smokers and nonsmokers (P = 0.06). For mothers who smoked during pregnancy, 11.3% of their children required continuous positive airway pressure at 36 weeks PMA, whereas only 5.7% of children whose mothers did not smoke required the treatment. The median number of days on mechanical ventilation for a child of a maternal smoker was greater than that for infants born from nonsmoker mothers (14 vs. 6 d; P = 0.04). As a sensitivity analysis, we evaluated whether the association with smoking remained between the four-category BPD variable (none, mild, moderate, and severe). The risk was similar between the groups that were combined (see online supplement). When we used an ordinal model for multinomial data (four-category BPD), maternal smoking remained significant after adjusting for the other covariates (OR, 1.7; 95% CI, 1.03–2.81; P = 0.04). We evaluated many potential interactions with the smoking variable, but none of the interactions reached statistical significance (see online supplement). Variables were selected for inclusion in the model on the basis of a priori knowledge of clinical importance.
Table 4.
Postnatal Neonatal Intensive Care Unit Events by Maternal Smoking Status
| Postnatal NICU Events (n = 587) | Smokers (n = 80) | Nonsmokers (n = 507) | P Value |
|---|---|---|---|
| Pneumonia | 10 (12.5%) | 48 (9.5%) | 0.40 |
| Necrotizing enterocolitis | 10 (12.5%) | 56 (11.0%) | 0.70 |
| Sepsis | 16 (20%) | 93 (18.3%) | 0.72 |
| Threshold retinopathy | 9 (11.3%) | 38 (7.5%) | 0.25 |
| Days of CPAP | 12.5 (4.5–23) | 14 (6–29) | 0.13 |
| Required CPAP at 36 wk PMA | 9 (11.3%) | 29 (5.7%) | 0.06 |
| Days of MV | 14 (2–40) | 6 (2–21) | 0.04 |
| Required MV at 36 wk PMA | 7 (8.8%) | 23 (4.5%) | 0.44 |
| Length of stay in NICU | 91.5 (74–118) | 85 (70–110) | 0.11 |
| Discharged on oxygen | 48 (60.0%) | 293 (57.8%) | 0.71 |
| Mortality | 2 (2.5%) | 6 (1.2%) | 0.35 |
| Total oxygen days (includes 1-yr follow-up) | 73 (39.5–110.5) | 73 (43.5–98) | 0.68 |
Definition of abbreviations: CPAP = continuous positive airway pressure; MV = mechanical ventilation; NICU = neonatal intensive care unit; PMA = postmenstrual age.
Descriptive statistics [count (percent) or median (interquartile range)] and univariate comparisons are presented for postnatal neonatal intensive care unit events, stratified by maternal smoking status.
Follow-up surveys were used to examine risk factors for continuing respiratory problems. Five hundred thirty-seven of the 587 children (91.5%) completed one or more surveys. Of these, 223 completed all four, 134 completed three, 110 completed two, and 70 completed only one. Of 537 children with at least one survey, 372 infants (69.3%) had late respiratory disease during early childhood, as defined above. There were 13 children (2.4%) whose respiratory diagnosis could not be computed, owing to missing questions. Similar BPD rates (44% vs. 41%), maternal ages (mean, 27.2 vs. 27.8 yr), and gestational ages (mean, 26.6 vs. 27.0 wk) were observed for the subjects who had a missing respiratory diagnosis. More of the subjects with missing respiratory diagnoses than those with respiratory diagnosis information were smokers (22% vs. 13%; P = 0.04). By univariate analysis, there were significant differences in gestational age (P < 0.01) and maternal age (P = 0.01) between those who had a late respiratory diagnosis and those who did not (Table 5). There was a higher percentage of children with a late respiratory diagnosis who were from black or African American parents (P < 0.01) than those without a diagnosis. There was a lower percentage of white children who were diagnosed with a late respiratory diagnosis than those who were not (P < 0.01). Children of mothers who smoked during pregnancy were more likely to develop a respiratory disorder (P = 0.02) (Table 6). Pregnancies complicated by chorioamnionitis had a higher rate of children with a late respiratory diagnosis than those that did not (P = 0.03). Children who were given a surfactant in the delivery room were more likely to develop a respiratory disorder (P < 0.01), and those with threshold retinopathy of prematurity had a higher diagnosis rate (P = 0.01).
Table 5.
Subject Characteristics by Respiratory Diagnosis Status
| No Respiratory Diagnosis (n = 152) | Respiratory Diagnosis (n = 372) | P Value | |
|---|---|---|---|
| Birth weight, g | 970.5 (815 to 1,092.5) | 910 (756 to 1,085) | 0.14 |
| Birth weight z-score | −0.399 (−1.04 to 0.24) | −0.215 (−0.76 to 0.29) | 0.06 |
| Birth weight strata, g | |||
| 500–749 | 29 (19.1%) | 87 (23.4%) | 0.28 |
| 750–999 | 57 (37.5%) | 140 (37.6%) | 0.98 |
| 1,000–1,250 | 66 (43.4%) | 145 (39.0%) | 0.35 |
| Small for gestational age | 52 (34.2%) | 89 (23.9%) | 0.02 |
| Birth length, cm | 35.45 (33 to 37) | 35 (32 to 37) | 0.03 |
| Birth head circumference, cm | 25 (23.5 to 26.5) | 24.5 (23 to 26) | <0.01 |
| Gestational age, wk | 27 (26 to 29) | 27 (25 to 28) | <0.01 |
| Maternal age, yr | 29 (25 to 33) | 28 (22 to 32) | 0.01 |
| Male sex | 71 (46.7%) | 192 (51.6%) | 0.31 |
| Maternal race | |||
| Asian | 3 (2.0%) | 4 (1.1%) | 0.42 |
| Black or African American | 18 (11.8%) | 92 (24.7%) | <0.01 |
| Hawaiian or Pacific Islander | 0 (0%) | 0 (0%) | — |
| White | 130 (85.5%) | 273 (73.4%) | <0.01 |
| Other | 0 (0%) | 1 (0.3%) | — |
| Unknown | 1 (0.7%) | 2 (0.5%) | 0.868 |
| Maternal ethnicity | |||
| Hispanic or Latino | 30 (19.7%) | 69 (18.5%) | 0.75 |
| Not Hispanic or Latino | 122 (80.3%) | 302 (81.2%) | 0.81 |
| Multiple gestations | 37 (24.3%) | 98 (26.3%) | 0.63 |
| Antenatal corticosteroids | 124 (81.6%) | 312 (83.9%) | 0.75 |
| Cesarean section delivery | 104 (68.4%) | 262 (70.4%) | 0.65 |
| Intubated in delivery room | 85 (55.9%) | 239 (64.2%) | 0.06 |
| Intubated in NICU | 34 (22.4%) | 76 (20.4%) | 0.87 |
| Surfactant in delivery room | 103 (67.8%) | 308 (82.8%) | <0.01 |
| PDA medical treatment | 62 (40.8%) | 149 (40.1%) | 0.92 |
| PDA surgical ligation | 16 (10.5%) | 48 (12.9%) | 0.23 |
| IVH grade 3 or 4 | 6 (3.9%) | 15 (4.0%) | 0.96 |
| Threshold ROP | 4 (2.6%) | 37 (9.9%) | 0.01 |
| BPD severity | |||
| None or mild | 105 (69.1%) | 205 (55.1%) | <0.01 |
| Moderate or severe | 47 (30.9%) | 167 (44.9%) | <0.01 |
| Oxygen use at discharge, yes (%) | 90 (59.2%) | 209 (56.2%) | 0.53 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; IVH = intraventricular hemorrhage; NICU = neonatal intensive care unit; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity.
Descriptive statistics [count (percent) or median (interquartile range)] and univariate comparisons for the study demographics, stratified by respiratory diagnosis over the first 2 years of life.
Table 6.
Prenatal Factors by Respiratory Diagnosis Status
| Maternal Complications | No Late Respiratory Diagnosis | Late Respiratory Diagnosis | P Value |
|---|---|---|---|
| Preexisting diabetes | 3 (2%) | 19 (5.1%) | 0.11 |
| Gestational diabetes | 7 (4.6%) | 30 (8.1%) | 0.18 |
| Preexisting hypertension | 19 (12.5%) | 46 (12.4%) | 0.89 |
| Prolonged rupture of membranes | 26 (17.1%) | 72 (19.4%) | 0.51 |
| Chorioamnionitis | 20 (13.2%) | 79 (21.2%) | 0.03 |
| Preeclampsia | 46 (30.3%) | 95 (25.5%) | 0.25 |
| Antepartum hemorrhage | 14 (9.2%) | 47 (12.6%) | 0.30 |
| Maternal smoking | 11 (7.2%) | 55 (14.8%) | 0.02 |
| Maternal alcohol use | 3 (2.0%) | 8 (2.2%) | 0.90 |
| Maternal substance abuse | 7 (4.6%) | 37 (9.9%) | 0.05 |
Descriptive statistics [count (percent)] and univariate comparisons are presented for antenatal events, stratified by respiratory diagnosis over the first 2 years of life.
Of the children diagnosed with a respiratory disease in the first 2 years of life, 45% had moderate or severe BPD (Table 5). Similarly, of the children who were not diagnosed with a respiratory disease, 69% had no or mild BPD. A logistic regression was fitted for respiratory diagnosis over the first 2 years of life with dichotomized BPD as a predictor (c-index, 0.57). Children with moderate or severe BPD had a 1.82-fold increase in the odds of a respiratory diagnosis during the first 2 years of life (95% CI, 1.22–2.72; P < 0.01). In addition, a logistic regression was fitted for a respiratory diagnosis over the first 2 years of life with the four-category BPD as a predictor (c-index, 0.63). Children with severe BPD had a 5.0-fold increase in the odds of a respiratory diagnosis compared with those without BPD during the first 2 years of life (95% CI, 2.54–9.68; P < 0.01). Children with moderate BPD had a 1.64-fold increase in the odds of a respiratory diagnosis (95% CI, 0.95–2.83; P = 0.08), and children with mild BPD had 1.9-fold increase in the odds of a respiratory diagnosis compared with those without BPD (95% CI, 1.15–3.01; P = 0.01). In comparison with infants with no or mild BPD, infants with moderate or severe BPD had significantly more emergency room visits, hospitalizations, and respiratory medication use during the first 2 years of life (Table 7). Importantly, of those infants diagnosed with moderate or severe BPD, 22% had no clinical evidence of late respiratory disease after NICU discharge during early childhood. Of infants without BPD, 34% were not diagnosed with late respiratory disease postdischarge. The odds associated with mild BPD were slightly higher than the odds for moderate BPD, although this difference was not significant (P = 0.62).
Table 7.
Relationship of Bronchopulmonary Dysplasia Status to Late Respiratory Disease
| No or Mild BPD | Moderate or Severe BPD | P Value | |
|---|---|---|---|
| Number of surveys completed | 3 (2–4) | 3 (2–4) | 0.31 |
| Last survey completed, age, mo | 24 (18–24) | 24 (18–24) | 0.94 |
| Number of children with one or more emergency department visits | 167 (48.4%) | 142 (58.7%) | 0.01 |
| Number of children with one or more emergency department visits for respiratory reasons | 120 (34.8%) | 106 (43.8%) | 0.10 |
| Number of children with one or more hospitalizations | 96 (27.8%) | 98 (40.5%) | <0.01 |
| Number of children with one or more hospitalizations for respiratory reasons | 64 (18.6%) | 75 (31.0%) | <0.01 |
| Diuretic use | 5 (1.4%) | 13 (5.4%) | 0.01 |
| Bronchodilator use | 87 (25.2%) | 99 (40.9%) | <0.01 |
| Inhaled steroid use | 46 (13.3%) | 58 (24.0%) | <0.01 |
| Age when successfully taken off oxygen, d | 59 (32–107.5) | 180 (98.5–326) | <0.01 |
Definition of abbreviation: BPD = bronchopulmonary dysplasia.
Data are presented as count (percent) or median (interquartile range).
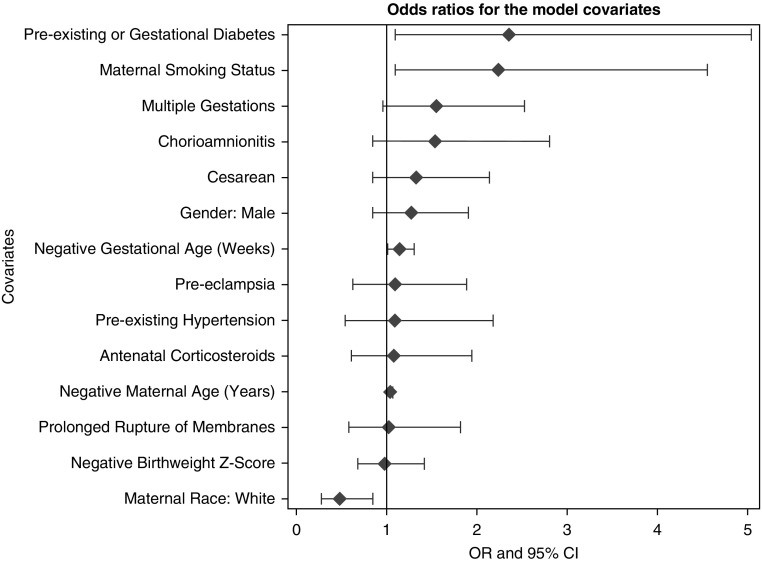
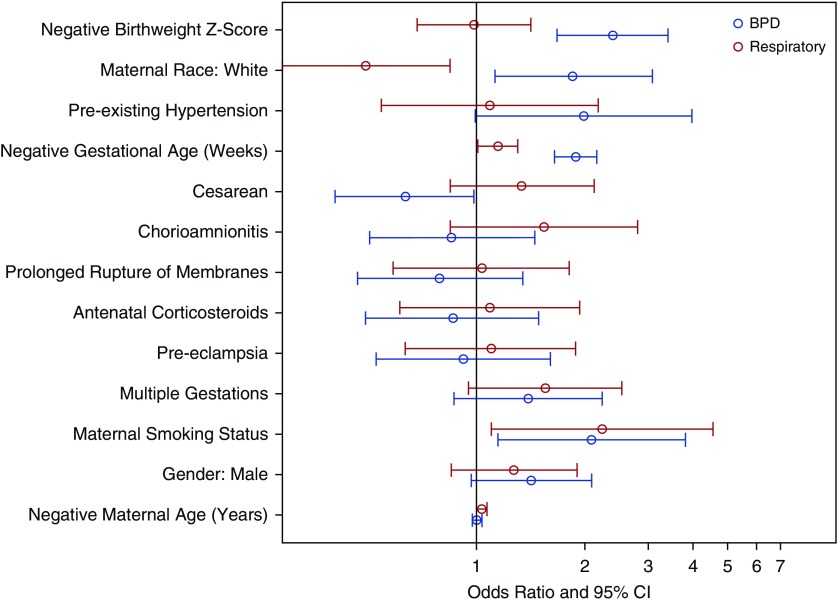
A multiple linear logistic regression was fitted on respiratory diagnosis using a subset of predictors from the previous model of BPD status (Table 8) (c-index, 0.66). A subset of the predictors included for the BPD outcome was used for modeling late respiratory diagnosis owing to the differences in event rate. Included variables were selected a priori on the basis of clinical importance. There was a significant association between mothers smoking during pregnancy and children with a respiratory diagnosis during the first 2 years of life (P = 0.02) (Table 6). After adjusting for covariates, we found that children of mothers who had smoked had increased odds of a respiratory diagnosis of 2.18 (95% CI, 1.07–4.44; P = 0.03). Children of white mothers had a 0.51-fold decrease in the odds of late respiratory disease compared with those of other races (95% CI, 0.30–0.87; P = 0.01). Lower gestational age (OR, 1.15; 95% CI, 1.01–1.30; P = 0.04) was significantly associated with an increase in the odds of a late respiratory diagnosis. Children of mothers who had multiple gestations had a 1.68-fold increased odds of late respiratory disease (95% CI, 1.02–2.77; P = 0.04). Children of mothers who had preexisting diabetes or gestational diabetes had a 2.53-fold increase in odds of a late respiratory diagnosis compared with those who did not (95% CI, 1.16–5.54; P = 0.02). Lower maternal age (OR, 1.04; 95% CI, 1.00–1.08; P = 0.03) was significantly associated with an increase in the odds of a late respiratory diagnosis. All other covariates had insignificant ORs. The ORs from the model are ranked graphically in Figure 2. Figure 3 displays the common ORs from the full model predicting BPD status stacked with the OR from this model. Finally, children with late respiratory disease were more frequently exposed to passive smoking in the household than those without late respiratory disease (Table 9). In addition, exposure to household pets was less common in children with late respiratory disease.
Table 8.
Model Results of Logistic Regression for Respiratory Diagnosis
| Parameter | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Decreasing birth weight z-score, 1 SD | 1.04 | 0.72–1.50 | 0.85 |
| Male sex | 1.22 | 0.81–1.83 | 0.34 |
| Maternal race: white | 0.51 | 0.30–0.87 | 0.01 |
| Decreasing gestational age, wk | 1.15 | 1.01–1.30 | 0.04 |
| Maternal smoking status | 2.18 | 1.07–4.44 | 0.03 |
| Antenatal corticosteroids | 1.11 | 0.62–1.98 | 0.73 |
| Multiple gestations | 1.68 | 1.02–2.77 | 0.04 |
| Cesarean section delivery | 1.11 | 0.69–1.80 | 0.67 |
| Preexisting or gestational diabetes | 2.53 | 1.16–5.54 | 0.02 |
| Preexisting hypertension | 1.15 | 0.57–2.30 | 0.70 |
| Prolonged rupture of membranes | 0.97 | 0.55–1.71 | 0.91 |
| Chorioamnionitis | 1.56 | 0.85–2.83 | 0.15 |
| Preeclampsia | 1.11 | 0.64–1.92 | 0.72 |
| Decreasing maternal age, yr | 1.04 | 1.00–1.08 | 0.03 |
Risk factors for a respiratory diagnosis over first 2 years of life. Parametric estimates, Wald 95% confidence intervals, and P values derived from the logistic regression modeling analysis for a respiratory diagnosis over the first 2 years of life.
Figure 2.
Odds ratios (ORs) with 95% confidence intervals (CIs) for model covariates from the logistic regression analysis modeling respiratory diagnosis over the first 2 years of life.
Figure 3.
Comparisons of associations between perinatal factors with bronchopulmonary dysplasia (BPD) and late respiratory disease models. CI = confidence interval.
Table 9.
Environmental Exposures to Passive Smoking and Pets in Subjects with or without Late Respiratory Diagnosis
| No Late Respiratory Diagnosis | Respiratory Diagnosis | P Value | |
|---|---|---|---|
| PFT performed | 20 (13.2%) | 33 (8.9%) | 0.14 |
| RSV shots | 125 (82.2%) | 326 (87.6%) | 0.11 |
| Flu shots | 130 (85.5%) | 324 (87.1%) | 0.63 |
| Smoking allowed in child’s home | 4 (2.6%) | 27 (7.3%) | 0.04 |
| One or more people in the home smoke | 33 (21.6%) | 127 (37.7%) | <0.01 |
| Primary caregivers smoke occasionally or daily | 42 (27.6%) | 129 (38.6%) | 0.02 |
| Families with pets | 83 (54.6%) | 168 (45.2%) | 0.05 |
| Families with dogs | 64 (42.1%) | 145 (39.0%) | 0.51 |
| Families with cats | 32 (21.1%) | 66 (17.7%) | 0.38 |
| Families with other pets | 13 (8.6%) | 27 (7.3%) | 0.61 |
Definition of abbreviations: PFT = pulmonary function testing; RSV = respiratory syncytial virus.
Discussion
To better understand the contribution of antenatal factors to the development of BPD and late respiratory disease during early childhood, we performed a prospective longitudinal study of preterm infants that included extensive data collection of perinatal, NICU, and postdischarge information. We report a striking association between maternal smoking and the subsequent diagnosis of moderate and severe BPD. After adjusting for covariates, we found that maternal smoking prior to birth increased the odds of having an infant with moderate or severe BPD by 2.02-fold. Maternal smoking was also associated with prolonged need for mechanical ventilation and the use of respiratory support during the NICU admission, as well as with late respiratory disease during infancy. Additional risk factors for BPD in this cohort also included lower gestational age at birth, lower birth weight z-scores, white race, and preexisting maternal hypertension. As shown in Table 3, decreasing birth weight z-score was an even better predictor of the development of BPD than maternal smoking. More infants with moderate and severe BPD had severe intraventricular hemorrhage and more often were treated with surgical ligation of a patent ductus arteriosus than were those with no or mild BPD. Overall, these findings suggest that maternal smoking and hypertension increase the risk for developing BPD after preterm birth and that maternal smoking and chorioamnionitis are strongly associated with an increased risk for late respiratory morbidities during early childhood.
To address ongoing controversies regarding how well the current National Institutes of Health definition of BPD at 36 weeks PMA reflects the risk for persistent respiratory disease during childhood (10, 11), we further examined the relationship between the diagnosis of moderate or severe BPD and late respiratory outcomes during infancy. In this cohort, infants with BPD had significantly more emergency room visits, hospitalizations, and respiratory medication use during the first 2 years of life than those without BPD. Interestingly, 22% of infants who were diagnosed with moderate or severe BPD at 36 weeks PMA and 34% without BPD had no clinical evidence of late respiratory disease after NICU discharge during early childhood.
While recognizing the multifactorial etiologies of BPD, we found that the link between smoking and BPD risk in our study cohort to be striking. Previous studies have shown that maternal smoking is strongly associated with premature birth, low birth weight, and abnormal infant lung function (32, 33). Factors associated with BPD, such as preeclampsia, preexisting maternal hypertension, and demographic factors, were adjusted for in the model to avoid confounders. Maternal smoking during pregnancy is a known risk factor for impaired childhood lung function and respiratory disease after term birth (34–36). Pooled analyses of several birth cohort studies showed that maternal smoking during pregnancy independently increased the risk of asthma at 4–6 years of age by 39 to 65% (35). Possible mechanisms include increased inflammatory cytokine production, altered placental function, or direct impact on lung development, which disrupts lung structure and function (37, 38). In addition, critical interactions between in utero events with asthma susceptibility genes suggest a potential mechanism for gene-by-environment interactions that may contribute to high risk for disease (39). We lack quantitative data to address potential dose–response relationships between the amount of smoking and respiratory outcomes in our population.
Hypertensive disorders of pregnancy, especially preeclampsia, have previously been associated with high risk for BPD after preterm birth. In a small cohort study, the risk for BPD was dramatically increased in the presence of preeclampsia, even after accounting for intrauterine growth restriction (IUGR) (18). This finding has been confirmed in some studies (40) but not in others (41). Our findings extend previous observations by providing further evidence for antenatal events that are strongly associated with respiratory disease in preterm infants throughout early childhood. These data are of further interest because they were obtained from a prospective longitudinal study that included strict characterization of BPD definition and assessment of its severity along with the inclusion of follow-up assessments throughout the first 2 years of life to examine links between BPD severity and respiratory outcomes during infancy.
Mechanisms through which antenatal events contribute to high risk for BPD or late respiratory disease in childhood are at least partly related to placental abnormalities, as observed in hypertensive disorders of pregnancy, preeclampsia, chorioamnionitis, and other disorders (11, 42, 43). Animal studies have shown that prenatal insults can be sufficient to impair lung structure during infancy, even without adverse postnatal stimuli (44–46). Researchers in clinical studies have reported striking associations between placental histopathology and the presence of IUGR, as well as high risk for BPD and BPD with pulmonary hypertension (42, 43). Preterm human infants with IUGR have consistently been shown have a high risk for BPD, providing strong proof regarding the importance of fetal events in the pathobiology of BPD (15, 47). In addition to the presence or absence of BPD at 36 weeks, preterm infants who were born with IUGR remained at high risk for abnormal lung function at school age (48, 49).
Potential limitations of the present study include that maternal smoking was reported by the patient rather than through biochemical assays for nicotine exposure (50). We have no evidence that underreporting rates differed between study groups in our cohort. In addition, we did not perform pathologic or histologic assessments of the placenta to better define chorioamnionitis or vascular pathology.
In conclusion, we found that maternal smoking and hypertension increased the risk for developing BPD after preterm birth and that maternal smoking is strongly associated with increased risk for late respiratory morbidities during early childhood. Twenty-two percent of infants diagnosed with BPD and 34% of preterm infants without BPD had no clinical signs of late respiratory disease during early childhood, suggesting that factors beyond the BPD diagnosis alone modulate respiratory outcomes in childhood.
Footnotes
Supported by National Institutes of Health, NHLBI grant R01 HL085703, “Genetic Basis for Impaired Angiogenesis in BPD” (S.H.A., principal investigator).
Author Contributions: Conception and design: all authors; drafting of the manuscript: L.A.M., B.D.W., P.M.M., S.H.A., B.B.P., and J.D.; analysis and data interpretation: L.A.M. and B.D.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201612-2414OC on March 1, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP, Wyckoff M, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis PG, Doyle LW, Asztalos EV, Opie G, Bairam A, Solimano A, Arnon S, Sauve RS Caffeine for Apnea of Prematurity (CAP) Trial Investigators. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167:982–986.e2. doi: 10.1016/j.jpeds.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Abman SH. Bronchopulmonary dysplasia. New York: Informa HealthCare; 2010. [Google Scholar]
- 7.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunville CF, Sontag MK, Stratton KA, Ranade DJ, Abman SH, Mourani PM. Scope and impact of early and late preterm infants admitted to the PICU with respiratory illness. J Pediatr. 2010;157:209–214.e1. doi: 10.1016/j.jpeds.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11(Suppl 3):S146–S153. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, Reynolds AM, Shaw PA, Jobe AH Prematurity and Respiratory Outcomes Program. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. 2015;12:1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manuck TA, Levy PT, Gyamfi-Bannerman C, Jobe AH, Blaisdell CJ. Prenatal and perinatal determinants of lung health and disease in early life. JAMA Pediatr. 2016;170:e154577. doi: 10.1001/jamapediatrics.2015.4577. [DOI] [PubMed] [Google Scholar]
- 12.Farstad T, Bratlid D, Medbø S, Markestad T Norwegian Extreme Prematurity Study Group. Bronchopulmonary dysplasia: prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr. 2011;100:53–58. doi: 10.1111/j.1651-2227.2010.01959.x. [DOI] [PubMed] [Google Scholar]
- 13.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, Stoll BJ, Buchter S, Laptook AR, Ehrenkranz RA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wai KC, Kohn MA, Ballard RA, Truog WE, Black DM, Asselin JM, Ballard PL, Rogers EE, Keller RL Trial of Late Surfactant (TOLSURF) Study Group. Early cumulative supplemental oxygen predicts bronchopulmonary dysplasia in high risk extremely low gestational age newborns. J Pediatr. 2016;177:97–102.e2. doi: 10.1016/j.jpeds.2016.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, Ehrenkranz RA, Boggess K, Leviton A Extremely Low Gestational Age Newborn Study Investigators. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124:e450–e458. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 17.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C Developmental Epidemiology Network Investigators. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 18.Hansen AR, Barnés CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010;156:532–536. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson L, Haglund B, Odlind V, Altman M, Ewald U, Kieler H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015;104:259–263. doi: 10.1111/apa.12888. [DOI] [PubMed] [Google Scholar]
- 20.Jobe AH. Effects of chorioamnionitis on the fetal lung. Clin Perinatol. 2012;39:441–457. doi: 10.1016/j.clp.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikström AK, Granath F. Maternal obesity and risk of preterm delivery. JAMA. 2013;309:2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 22.Gemmell L, Martin L, Murphy KE, Modi N, Håkansson S, Reichman B, Lui K, Kusuda S, Sjörs G, Mirea L, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks’ gestation. J Perinatol. 2016;36:1067–1072. doi: 10.1038/jp.2016.133. [DOI] [PubMed] [Google Scholar]
- 23.Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Källén K. The impact of maternal smoking during pregnancy on delivery outcome. Eur J Public Health. 2001;11:329–333. doi: 10.1093/eurpub/11.3.329. [DOI] [PubMed] [Google Scholar]
- 25.McEvoy CT, Spindel ER. Pulmonary effects of maternal smoking on fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev. 2017;21:27–33. doi: 10.1016/j.prrv.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegler J, Jensen R, Segerer H, Ehlers S, Kühn T, Jenke A, Gebauer C, Möller J, Orlikowsky T, Heitmann F, et al. Influence of smoking and alcohol during pregnancy on outcome of VLBW infants. Z Geburtshilfe Neonatol. 2013;217:215–219. doi: 10.1055/s-0033-1361145. [DOI] [PubMed] [Google Scholar]
- 27.Isayama T, Shah PS, Ye XY, Dunn M, Da Silva O, Alvaro R, Lee SK. Adverse impact of maternal cigarette smoking on preterm infants. Am J Perinatol. 2015;32:1105–1111. doi: 10.1055/s-0035-1548728. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–456. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 30.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd ed. New York: Springer; 2015. [Google Scholar]
- 32.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, Bunten C, Leiva M, Gonzales D, Hollister-Smith J, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311:2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–705. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
- 34.Hollams EM, de Klerk NH, Holt PG, Sly PD. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189:401–407. doi: 10.1164/rccm.201302-0323OC. [DOI] [PubMed] [Google Scholar]
- 35.Neuman Å, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, Gehring U, Granell R, Henderson J, Heinrich J, et al. ENRIECO Consortium. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186:1037–1043. doi: 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- 36.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 37.Macaubas C, de Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, Sly PD, Holt PG. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 2003;362:1192–1197. doi: 10.1016/s0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- 38.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- 39.Scholtens S, Postma DS, Moffatt MF, Panasevich S, Granell R, Henderson AJ, Melén E, Nyberg F, Pershagen G, Jarvis D, et al. GABRIELA study group. Novel childhood asthma genes interact with in utero and early-life tobacco smoke exposure. J Allergy Clin Immunol. 2014;133:885–888. doi: 10.1016/j.jaci.2013.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozkan H, Cetinkaya M, Koksal N. Increased incidence of bronchopulmonary dysplasia in preterm infants exposed to preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2681–2685. doi: 10.3109/14767058.2012.708371. [DOI] [PubMed] [Google Scholar]
- 41.O’Shea JE, Davis PG, Doyle LW Victorian Infant Collaborative Study Group. Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res. 2012;71:210–214. doi: 10.1038/pr.2011.27. [DOI] [PubMed] [Google Scholar]
- 42.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33:553–557. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, Su E, Porta N, Gotteiner N, Ernst LM. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35:570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rozance PJ, Seedorf GJ, Brown A, Roe G, O’Meara MC, Gien J, Tang JR, Abman SH. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;301:L860–L871. doi: 10.1152/ajplung.00197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang JR, Seedorf GJ, Muehlethaler V, Walker DL, Markham NE, Balasubramaniam V, Abman SH. Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2010;299:L735–L748. doi: 10.1152/ajplung.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lal MK, Manktelow BN, Draper ES, Field DJ. Chronic lung disease of prematurity and intrauterine growth retardation: a population-based study. Pediatrics. 2003;111:483–487. doi: 10.1542/peds.111.3.483. [DOI] [PubMed] [Google Scholar]
- 48.Ronkainen E, Dunder T, Kaukola T, Marttila R, Hallman M. Intrauterine growth restriction predicts lower lung function at school age in children born very preterm. Arch Dis Child Fetal Neonatal Ed. 2016;101:F412–F417. doi: 10.1136/archdischild-2015-308922. [DOI] [PubMed] [Google Scholar]
- 49.Greenough A, Yuksel B, Cheeseman P. Effect of in utero growth retardation on lung function at follow-up of prematurely born infants. Eur Respir J. 2004;24:731–733. doi: 10.1183/09031936.04.00060304. [DOI] [PubMed] [Google Scholar]
- 50.Hall ES, Wexelblatt SL, Greenberg JM. Self-reported and laboratory evaluation of late pregnancy nicotine exposure and drugs of abuse. J Perinatol. 2016;36:814–818. doi: 10.1038/jp.2016.100. [DOI] [PubMed] [Google Scholar]