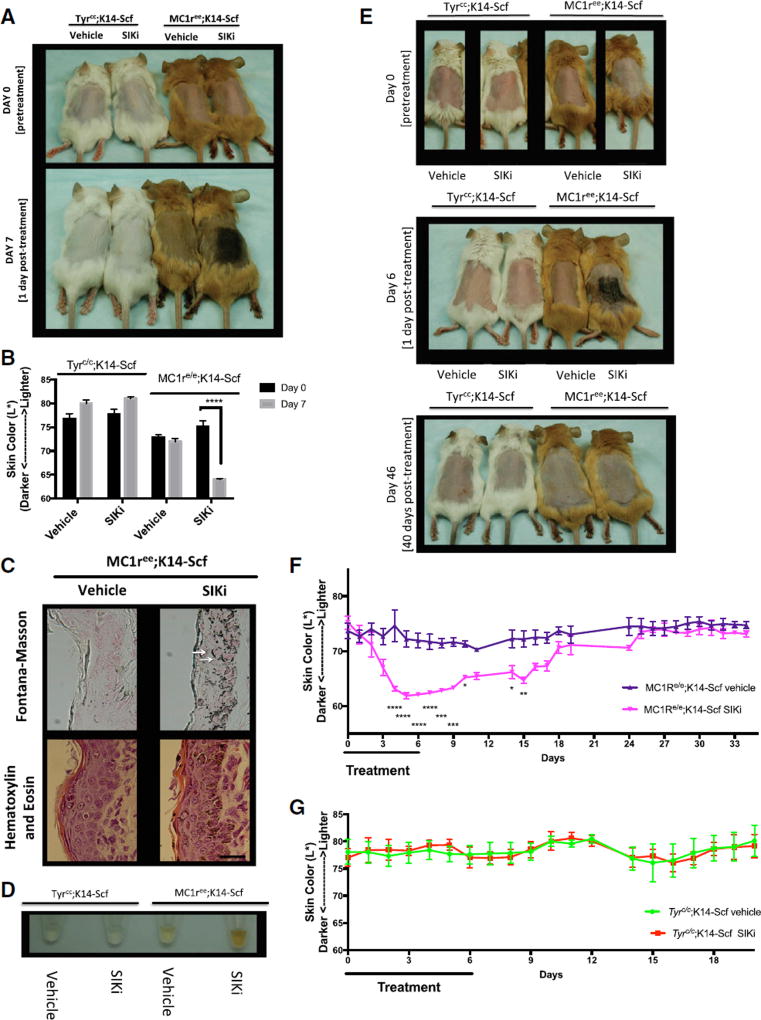
Figure 2. Topical Treatment with HG9-91-01 Causes Robust Darkening that Is Progressive and Reversible in Mc1re/e;K14-SCF Mice.
(A–D) Shown here: (A) Mc1re/e;K14-SCF mice and Tyrc/c;K14-SCF mice before treatment (day 0) and after 7 days of treatment (day 7) with 30 µL vehicle control (70% ethanol, 30% propylene glycol) or 37.5 mM HG 9-91-01 (image is representative of n = 4 experiments). (B) Reflective colorimetry measurements (L* white-black color axis; n = 4, mean ± SEM) and (D) melanin extraction (image is representative of n = 4 experiments) of the Mc1re/e;K14-SCF mice and Tyrc/c;K14-SCF mice described in (A). (C) Skin sections of Mc1re/e;K14-SCF mice described in (A) stained with Fontana-Masson (eumelanin) (top two panels) or H&E (bottom two panels); (magnification, 400×). White arrows represent nuclear capping; scale bar represents 25 µm.
(E) Mc1re/e;K14-SCF mice and Tyrc/c;K14-SCF mice before treatment (day 0) and after 6 days of treatment with 30 µL vehicle control (70% ethanol, 30% propylene glycol) or 37.5 mM HG 9-91-01 (day 6), and 40 days post-treatment (day 46) (vehicle mouse in day-46 photo is different from that in the day-0 and day-6 photos).
(F and G) Reflective colorimetry measurements (CIE L* white-black color axis) of (F) Mc1re/e;K14-SCF mice and (G) Tyrc/c;K14-SCF mice treated as described in (E). Vehicle-treated Mc1re/e;K14-SCF mice: n = 5 (days 0–19), and n = 4 (days 24–34); HG 9-91-01-treated Mc1re/e;K14-SCF mice: n = 3; vehicle-treated Tyrc/c;K14-SCF mice: n = 3 (days 0–10), and n = 2 (days 11–20); HG 9-91-01-treated Tyrc/c;K14-SCF mice: n = 3 (mean ± SEM). For the graph in (B), statistical significance is reported as follows: ****p < 0.0001, multiple t test analysis with the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. For the graphs in (F) and (G), statistical significance is reported as follows: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001, two-way ANOVA with Sidak’s multiple comparisons test comparing treatment to vehicle control at each time point.