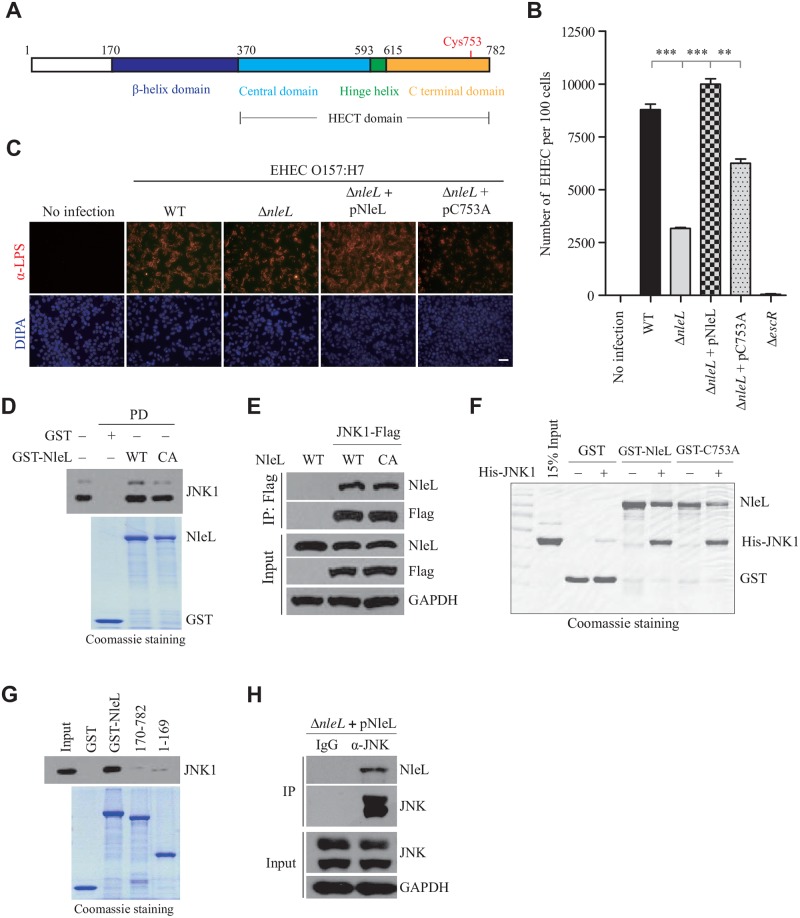
Fig 1. NleL contributes to EHEC attachment to host, and interacts with human JNK1 protein.
(A) Domain structure of NleL from E. coli O157:H7. Cys753 in the C-terminal domain of NleL is the catalytic site responsible for the E3 Ub ligase activity of NleL. (B) Quantification of EHEC O157 attached to HeLa cells by colony formation assay. HeLa cells were infected with indicated EHEC strains with multiplicity of infection (MOI) of 100:1 for 2.5 h, washed with PBS and then re-cultured 2 h in fresh DMEM medium. Infected HeLa cells were thoroughly washed with PBS and aseptically lysed in 1% Triton X-100 buffer. Then bacterial colonies were determined after HeLa lysate being plated onto LB agar. Bars represent mean ± s.d. from three biological replicates, ***P < 0.001 (Student’s t-test, n = 3). (C) NleL promoted attachment of EHEC to mammalian cells. Infected HEK293T cells were thoroughly washed with PBS and subjected to immunofluorescence microscopy with anti-LPS antibody (in red). Scale bar, 50 μm. (D) Interaction of GST-NleL with endogenous JNK1. GST–tagged wild type or C753A mutant of NleL (GST-NleL-CA) were mixed with HEK293T cell lysates and the bound proteins were immunoblotted with the antibody specific for JNK1. GST, GST-tagged wild-type NleL or its C753A mutant were expressed and purified from bacteria. The C753A mutant was enzymatically dead and generated by substitution of the active site Cys753 with Ala. (E) NleL interacted with JNK1 in vivo. Flag-tagged JNK1, His6-tagged NleL (His-NleL) or its C753A mutant (His-NleL-CA) were ectopically expressed in HEK293T cells. Cells were lysed and subjected to immunoprecipitation (IP) with anti-Flag beads, followed by immunoblotting (IB) analysis with indicated antibodies. (F) Human JNK1 interacted with NleL in vitro. His6-tagged JNK1 (His-JNK1), GST-tagged wild-type NleL or its C753A mutant were purified from bacteria. (G) GST pull-down assay of full-length NleL or its truncations with JNK1. GST-tagged full-length NleL or its truncation mutants were individually mixed with lysates of the HEK293T cells expressing JNK1. After pull-down, Flag-tagged JNK1 was detected by IB analysis with anti-Flag. (H) Flag-tagged NleL secreted by EHEC O157 interacted with JNK in HEK293T cells. HEK293T cells were infected by EHEC strain expressing Flag-tagged NleL. Infected cells were thoroughly washed with PBS and lysed in IP buffer, and then IP with anti-JNK antibody and IB with anti-Flag antibody were successively performed. Data shown here are representative of at least three independent experiments.