Abstract
Radiobromine labelled compounds can be used for positron emission tomography (PET) imaging (i.e. 76Br) and for radiation therapy (i.e. 77Br). However, the commonly used electrophilic substitution reaction using no-carrier-added (NCA) radiobromide does not always afford the desired product due to the high reactivity of the brominating intermediate. A nucleophilic substitution by bromide, such as radiobromination of iodonium precursors, provides an alternative route for the synthesis of bromo-radiopharmaceuticals. The applicability of aromatic radiobromination by nucleophilic substitution using diaryliodonium salt precursors was evaluated using iodonium model compounds and [76Br]/[77Br]bromide. Radiobromination was observed under all conditions tested, in up to quantitative yields. A QMA cartridge treatment method and a base-free method have been developed, and no extra base is needed for either method. The base-free conditions are mild and afford much cleaner reactions. Up to 20% water is tolerated in the reactions without reducing the radiochemical yields. NCA and carrier-added reactions afforded similar results. 4-Bromobenzaldehyde and 4-bromobenzoate have been radiosynthesized reliably and in good yields. These results indicate that this method is robust and efficient, and thus will provide a route for radiobromination of electron-deficient arenes and an alternative route for the synthesis of bromo-radiopharmaceuticals for biological evaluations.
Keywords: Labelling methods, radiobromination, diaryliodonium salts, bromine-76, bromine-77, Auger electron radiation, PET imaging
Graphical Abstract
The initial evaluation of nucleophilic radiobromination of arenes using diaryliodonium precursors and [76Br] or [77Br]bromide indicates that this method is robust and efficient. High incorporation was achieved with conventional heating or microwave irradiation under base-free conditions, and water and carrier-bromide were well tolerated in the reaction. Thus, this method could provide an alternative route for the synthesis of bromo-radiopharmaceuticals for biological evaluations.
Introduction
Halogen radioisotopes are commonly used in nuclear medicine for both imaging and radiation therapy. For example, 18F is the most widely used radioisotope for imaging using positron emission tomography (PET), and iodine radioisotopes are used for both imaging and therapy. Radiobromine, while not used as often as 18F and radioiodine isotopes, has some advantages over them.1,2 Fluorine has only one radioisotope that is useful only for PET imaging, but bromine has several useful isotopes that can be used for both PET imaging or for radiotherapy. In addition, bromine is smaller in size than iodine, and the C-Br bond is stronger than the C-I bond, leading to greater in vivo stability and potentially better pharmacokinetic and pharmacodynamic properties for brominated compounds. Unlike radioiodine, which often accumulates in the thyroid after in vivo metabolism of iodinated radiopharmaceuticals and thus can cause large radiation exposure to this organ, radiobromine-labelled compounds pose no such concerns.
Among the bromine radioisotopes, 75Br, 76Br and 77Br have promise for PET imaging and radiation therapy. 75Br (73% β+, 27% electron capture (EC)), with a half-life of 1.6 hours, is suitable for PET imaging. However, [76Br]bromide and [77Br]bromide can be produced more practically in high specific activity by the 76/77Se(p,n)76/77Br nuclear reaction on a 76Se- or 77Se-enriched Cu2Se target.4 76Br (55% β+, 45% EC), with a half-life of 16 hours, is used mainly for PET imaging; 77Br is an Auger emitter with a half-life of 57 hours and decays almost exclusively by EC (99%), a process that eventually produces Auger electrons.3 Auger electron radiation is highly toxic when the decay occurs in close proximity to DNA strands, while it is of little damage outside nucleus. Auger electrons from 77Br decay are as efficient in killing cells as densely ionizing alpha particles, and they compare favorably with the radiotoxic effects of DNA-bound 125I labelled compounds.5 Therefore, 76/77Br labelled compounds may make a theranostic pair for radiotherapy and PET imaging.
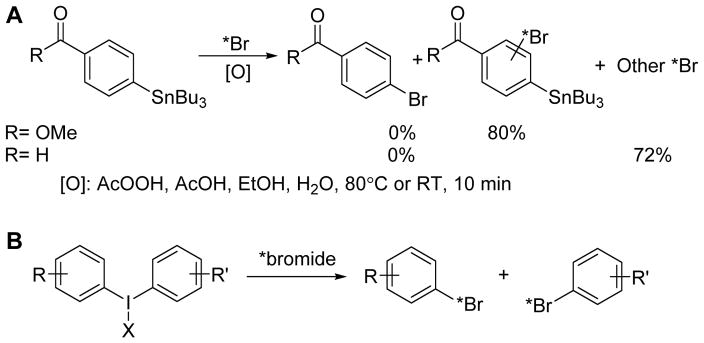
With respect to the chemistry of radiolabeling and compared with radiofluorination and radioiodination, radiobromination is the least studied radiohalogenation reaction. The chemical reactivity of bromine (Br2) is lower than that of fluorine but greater than that of iodine. Electrophilic substitution is commonly used for radiobromination. However, no-carrier-added (NCA) electrophilic bromination does not always afford the desired product due to the high reactivity of the brominating intermediate.6 As shown in our preliminary labeling studies (Scheme 1A), the incorporation of radiobromine was high, but only minimal amounts of the desired products, if any at all, were produced from electrophilic destannylation reactions of the tributyltin precursors under our optimized conditions. Either the final radioactive product was a very lipophilic compound with tributyltin still attached or multiple unknown products were produced from preferential radiobromination of reactive impurities present in the reaction mixture. These observations suggest that electrophilic radiobrominations, which are often considered to be straightforward reactions, can—in fact—be quite complicated.
Scheme 1.
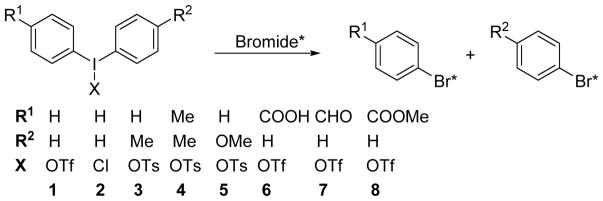
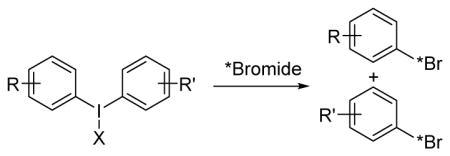
Significant progress has been made in nucleophilic radiofluorination of aromatic compounds, including the well explored route using diaryliodonium salts as precursors.7–9 Radiochlorination,10 radiobromination,11 radioiodonation,12,13 and radioastatination13 using diaryliodonium precursors have also been reported and shown to have promise for the labeling of aromatic compounds with radiohalogens. However, the report on radiobromination only demonstrated the feasibility of such reaction,11 and an in-depth exploration is needed. The general goal of the studies reported here is to evaluate the potential of using diaryliodonium salt precursors for nucleophilic radiobromination of radiopharmaceuticals to enable their evaluation for PET imaging and targeted radiotherapy (Scheme 1B).
Experimental
General information
Materials, Isotope Production and Purifications
All chemicals were obtained from standard commercial sources and used without further purification. All reactions were carried out by standard air-free and moisture-free techniques under an inert nitrogen atmosphere with dry solvents unless otherwise stated. 76Br and 77Br were produced at the Washington University cyclotron facility by the 76/77Se(p,n)76/77Br nuclear reaction on a 76Se- or 77Se-enriched Cu2Se target. 76Br and 77Br were recovered via a modified dry distillation method. The radionuclide was extracted with MQ water (Millipore Milli-Q water purification system purified water) and dispatched for use in MQ water. High performance liquid chromatography (HPLC) was performed with an ultraviolet detector and a well-scintillation NaI (Tl) detector and associated electronics for radioactivity detection. An Altima C18 250 × 4.6 mm 10 μm analytical column was used for analysis. Acetonitrile and water with 0.1% TFA were used as the HPLC mobile phase. Radio-TLC was accomplished using a Bioscan AR-2000 imaging scanner (Bioscan, Inc., Washington, DC). Published methods were used for the synthesis of compound 3,10 4,14 5,10 6,15 7,16 and 8.17
Drying procedure
Into a Wheaton V vial (5 mL) were loaded a solution of ammonium hydroxide (0.28%, 10 μL) and the radioactivity in MQ water. The vial was sealed firmly with an open top cap and a septum. Two 18G needles were inserted, one as argon inlet and one as a vent. A QMA Sep-Pak was used on the vent side to trap escaped radioactivity, if any, and the removed water was collected in a tube at room temperature. This setup is to prevent the contamination of the hood/hot cell with long-lived isotopes. The vial was inserted fully into a heating block (105–110 °C), and the flow was maintained to only just disturb the top of the liquid in the vial but sufficient to remove vapor from the vial. When the solvent was fully removed and the radioactivity was dry, the vial was allowed to cool to room temperature before further use. After DMF or DMSO was added to the vial, it was vortexed briefly.
Radiochemistry:QMA method
Radioactivity in water or methanol (1 mL) was loaded onto a Waters QMA Sep-Pak (46 mg, CO3), and then the Sep-Pak was rinsed with methanol (3 mL). Radioactivity was eluted from the column into a Pyrex tube (10 mL) using a solution of diaryliodonium precursor (5 mg) in methanol (0.5 mL), followed by rinsing the column with methanol (0.5 mL). After methanol was removed from the Pyrex tube under argon flow at 80 °C with a charcoal trap attached to the vent, DMSO (300 μL) was added to the residue. The reaction mixture was vortexed and heated at 130 °C for 10 or 30 min. Before opening, the tube was cooled to room temperature and vortexed. The reaction mixture was then analyzed by HPLC. In the cases when activity was eluted directly with a solution in DMSO or DMF, the QMA cartridge with trapped radioactivity was first rinsed with acetonitrile (1 mL) and DMSO (or DMF) (3×0.5 mL), and then the radioactivity was eluted into a Pyrex tube (10 mL) dropwise using a solution of a diaryliodonium precursor (5 mg) in DMSO (or DMF) (0.5 mL).
Radiochemistry: Base-free
An aliquot of radioactivity in MQ water, DMF or DMSO and a diaryliodonium precursor in DMF or DMSO were added to a Pyrex tube (10 mL). The tube was capped, vortexed and heated at the specified temperature. For microwave irradiation, the reaction mixture in a total volume of at least 500 μL was irradiated in a custom-made microwave chamber (60W) for 30 sec. Before opening for analysis, the tube was cooled down and vortexed. The reaction mixture was then analyzed by HPLC.
Results and discussion
[76Br] and [77Br]bromide
Because 76Br and 77Br are produced as bromide ion by exactly the same production and isolation procedures, their chemical behaviors are expected to be identical. Hence, for this study either [76Br]bromide or [77Br]bromide, depending on the availability of cyclotron production, was used for the radiobromination reactions below. Because bromide was processed in water (1–1.5 mL), the bromide is normally dried at elevated temperature under a flow of argon or nitrogen to facilitate the radiolabeling reaction, even for electrophilic radiobromination. However, unlike the drying of [18F]fluoride with potassium carbonate and Krytofix 222, improper drying of bromide may result significant loss of radioactivity. The best practice for drying bromide is described in the Experimental section. It typically takes 20–30 min to remove 1 mL of water by this method, and less than 5% of total radioactivity is lost. A Waters QMA SepPak was used as a trap/safe of bromide during the drying.
QMA-treatment method
Recently, a minimalist approach was reported for radiofluorination involving the use of a methanolic solution of diaryliodonium salts to elute radioactivity directly from a QMA column, without the need for azeotropic drying or the addition of extra base.16 We evaluated this method for radiobromination using some simple diaryliodonium compounds (1–6) (Scheme 2) in model reactions; the results are shown in Table 1.
Scheme 2.
Table 1.
Radiobromination of iodonium salts after elution from a QMA column
| Entry | R1/R2 | X | Elution1 (%) | 130 °C Yield (%)2
|
|
|---|---|---|---|---|---|
| 10 min | 30 min | ||||
| 1 | Me/Me (4) | OTs | 55 | 27 | 50 |
| 2 | H/H (1) | OTf | 94 | 53 | 91 |
| 3 | H/H (1) | OTf | 1001 | / | 92 |
| 4 | H/Me (3) | OTs | 73 | 24/12 | 43/24 |
| 5 | H/H (2) | Cl | 92 | 41 | 73 |
| 6 | H/OMe (5) | OTs | 55 | 19/0 | 47/0 |
| 7 | COOH/H (6) | OTf | 0 | / | / |
Notes:
Eluted with iodonium salts in methanol, except that entry 3 was eluted in DMSO;
Radiochemical yield of desired product was determined by HPLC analysis.
In all reactions, the desired brominated products were the major or only radioactive products. Also, the bromination reaction continued to proceed with longer reaction time, suggesting that significant levels of precursor still remained, presumably because in this protocol no extra base was added to the reaction; the diaryliodonium salts are base sensitive. Similar to the directing effect of substituents in radiofluorinations using diaryliodonium precursors, radiobromide ion reacted preferentially with the more electron-poor ring. Therefore, as with the radiofluorinations, it was possible to radiolabel an aromatic ring with electron-donating substituents by increasing the electron density of the other ring to a greater extent. The reaction is clean compared to base-added radiolabeling protocols. The major non-radioactive products are presumably the iodine-substituted compounds, according to HPLC analysis. Similar to the reports of in the minimalist approach, we found that tosylate salts were not efficient in eluting radioactivity from the QMA cartridge. For unknown reasons, we were not able to elute any radioactivity from the cartridge using the benzoic acid derivative (6).
According to the literature,16 the elution efficiency of [18F]fluoride from a QMA cartridge with iodonium precursors in anhydrous DMF or DMSO was poor (<30%). An alcoholic solution was required to elute [18F]fluoride efficiently from the cartridge. Bromide, however, is different from fluoride in that the latter has very high affinity for the silica-based QMA resin and extensive hydrogen bonding. Direct elution of radiobromide with 1 (5 mg) in DMSO (0.5 mL), hence, turned out to be quantitative (Table 1. Entry 3), and the labeling reaction is the same as when activity is eluted in methanol (Table 1. Entry 2). Direct elution in reaction solvents is practical for radiolabeling; however, caution still needs to be taken with the QMA-treatment method: the process may introduce non-radioactive bromide and chloride in the reaction mixture, resulting in significant reduction of specific activity because of the limited amount of radioactivity used and interference with HPLC purification due to the formation of chlorine analogues.
Base-free method
The minimalist approach using QMA cartridge benefited from the fact that no extra base was added to the reaction. Therefore, base-free radiobromination was further evaluated (Table 2). Bromide was dried and dissolved in organic solvents. The bromination reaction with 4 (Table 2, entry 1) is similar to that of QMA method (Table 1, entry 1), but afforded better yield at 30 min. When DMF was used as the solvent for 4, the yield improved significantly to 90% after only 10 min at 130 °C. Good yield was also obtained even when 1 mg of 4 was used (Table 2, entry 3).
Table 2.
Radiobromination of diaryl iodonium salts with bromide without base
| Entry | Precursor (5 mg)
|
Solvent (300 μL) | 130 °C Yield (%)1
|
||
|---|---|---|---|---|---|
| R1/R2 | X | 10 min | 30 min | ||
| 1 | Me/Me (4) | OTs | DMSO | 26 | 70 |
| 2 | Me/Me (4) | OTs | DMSO/DMF2 | 89 | 100 |
| 3 | Me/Me (4) | OTs3 | DMF | 67 | 78 |
| 4 | Me/Me (4) | OTs | DMF | 91 | 89 |
| 5 | Me/Me (4) | OTs | H2O/DMF4 | / | 83 |
| 6 | COOMe/H (8) | OTf | DMSO | 965 | 985 |
| 7 | COOH/H (6) | OTf | DMSO | 56/10 | 61/14 |
| 8 | COOH/H (6) | OTf | DMF | 57/13 | 64/18 |
Notes:
Radiochemical yield of desired product was determined by HPLC analysis;
40 μL DMSO + 300 μL DMF;
1 mg precursor;
KBr (0.75 mg) in water (30 μL) was mixed with radiobromide;
Total yield of two products.
In electrophilic bromination, carrier-added and non-carrier-added reactions often afford different results. As shown in Table 2, entry 5, addition of a large amount of KBr (0.75 mg, 6.3 μmol) with 4 (5 mg, 10.4 μmol) in a homogenous 1:10 water/DMF reaction mixture still afforded over 80% yield of desired product after 30 min at 130 °C. This result indicates the highly specific and efficient reaction between diaryl iodonium 4 and bromide, and the good tolerance of these reactions for water. For the electron-deficient substrate 8, the reaction went almost to completion in 10 min at 130 °C, implying a faster reaction with the aromatic ring having an electron-withdrawing substituent than an electron-donating substituent. Under our analytical HPLC conditions, both desired products co-eluted, so the ratio for this experiment was not determined. It worth noting that the radiobromination with iodonium precursors even went well with the benzoic acid derivative (6). Collectively, the results suggest that radiobromination using diaryliodonium precursors appears to be a robust method for radiobromination of arene systems.
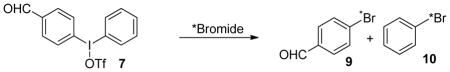
Radiosynthesis 4-bromobenzaldehyde (BBAL) using diaryliodonium precursor (7)
Radiolabelled 4-bromobenzaldehyde, as well as 4-bromobenzoic acid (BBA), are versatile building blocks that could be used as prosthetic groups for radiolabeling of variety of compounds. As shown in Scheme 1, the electrophilic reaction of the tributyltin precursor of benzaldehyde did not afford the desired product, most likely because the aromatic ring is deactivated for electrophilic bromination. There also have been no literature reports of the radiochemical synthesis of BBAL.
Because of the instability of BBAL towards high temperatures, some different temperatures were tested (Table 3). It appears that the optimal temperature is 130 °C, at which the yield at 10 min is similar to that at 30 min (Table 3, entry 2). Lower temperature with extended reaction times did not improve the reaction. Because microwave irradiation is often used in radiosyntheses to improve yields and shorten reaction times,18 we tested the radiobromination of 7 in our custom-made microwave chamber (60 W). The reaction mixture was quickly heated to boiling point of DMF, and the reaction was complete within 30 sec of microwave irradiation. Both BBAL (9) and bromobenzene (10) were formed in a ratio of 3:1, which is similar to the outcome under the optimal condition of conventional heating. The QMA-treatment method of direct elution of activity with a solution of iodonium salt afforded similar labelling results (Table 3. Entry 6). It is worth noting that radiofluorination of 7 using this method generated fluorobenzaldehyde exclusively in about 50% yield without any observable fluorobenzene, consistent with the higher regioselectivity of radiofluorination than radiobromination by the diaryliodonium approach.16
Table 3.
Radiosynthesis of 4-bromobenzaldehyde
| ||||
|---|---|---|---|---|
| Entry | Precursor (mg) | Solvent (μL) | Condition | Yield of 9/10 (%)1 |
| 1 | 5 | DMF (500) | 110 °C/30 min | 18/36 |
| 2 | 4 | DMF (300) | 130 °C/10 min | 45/16 |
| 130 °C/30 min | 49/16 | |||
| 3 | 3.5 | DMF (300) | 140 °C/12 min | 45/35 |
| 4 | 3.5 | DMF (600) | Microwave 30 sec | 70/26 |
| 5 | 1.9 | DMF (500) | Microwave 30 sec | 72/24 |
| 6 | 5 | DMF (500) | Microwave 30 sec | 64/302 |
Notes:
Yields of 4-bromobenzaldehyde/4-bromobenzene determined by HPLC;
QMA method was used to elute radioactivity with 7 in DMF (0.5 mL) in 90% efficiency.
Radiosynthesis of methyl 4-bromobenzoate using iodonium precursor (8)
The radiobromination of methyl 4-bromobenzoate (11) using diaryliodonium precursor (8) was carried out under microwave irradiation (Table 4). In DMF after 30 sec irradiation, the total yield of 11 and 10 was around 90%, even when only 0.5 mg of precursor was used. The ratio of 11 to 10 was 3:1 to 4:1, respectively. The reaction was driven to completion after 90 sec irradiation (Table 4, entry 2). Water was well tolerated in the reaction. This observation is the same as that for radiofluorination using iodonium precursors in aqueous solutions.19 When KBr (0.1 mg) was added as carrier, the yield remained high. When DMSO was used as the solvent, the reaction was complete after only 30 sec of microwave irradiation (Table 4, entry 7). This results most likely benefits from the high boiling point of DMSO. The QMA method of direct elution of activity in the reaction solvent also afforded very high yields (Table 4, entry 9).
Table 4.
Radiosynthesis of methyl 4-bromobenzoate

| ||||
|---|---|---|---|---|
| Entry | Precursor (mg) | H2O (μL) | Solvent (500 μL) | Microwave (30 sec)
|
| Yield (%) [Ratio]1 | ||||
| 1 | 5 | / | DMF | 70/23 |
| 2 | 2.5 | / | DMF | 69/232 |
| 3 | 0.5 | / | DMF | 70/18 |
| 4 | 2.5 | 50 | DMF | 64/25 |
| 5 | 2.5 | 100 | DMF | 63/19 |
| 6 | 2.5 | 1003 | DMF | 76/19 |
| 7 | 2.5 | / | DMSO | 75/22 |
| 8 | 4 | / | DMSO4 | 77/19 |
| 9 | 5 | / | DMF5 | 77/20 |
Notes:
Yield of methyl 4-bromobenzoate/bromobenzene by radioTLC and HPLC;
96% (+30 sec microwave), and 97% (+60 sec microwave);
With KBr (100 μg);
[6Br]bromide after 7 half-lives;
MQA method was used to elute radioactivity (0.5 mCi) with 8 in DMF (5×1 mg/0.1 mL) in 98% efficiency. Reaction condition: microwave 2×30 sec with interval of 30 sec.
In the past, oxidation of radiobromide ion to radiobromate by species from radiolysis in water over time was a concern, and this issue has neither been verified nor dismissed. When over 20 mCi [76Br]bromide was left in the original water and allowed to decay for 7 half-lives, the reactivity of [76Br]bromide was checked out by electrophilic and nucleophilic radiobrominations. No difference in reactivity was observed with freshly produced [76Br]bromide. The result of nucleophilic radiobromination using iodonium precursor was shown in Table 4, entry 8. This result suggests that the radioactivity is still in the form of radiobromide ion. Therefore, oxidation of radiobromide ion (at least for 76Br) to radiobromate should not be a concern; radiobromide ion differs from radioiodine isotopes in terms of the stability of chemical forms.20
Under the base-free labeling conditions, the reaction mixture is much cleaner than when base is added, according to HPLC analysis. As expected, iodine-substituted derivatives were observed as major by-products; however, they elute after the bromo-product on reversed-phase HPLC purification. Therefore, the radiobromine-labelled product can be easily purified by HPLC, and no impact on specific activity or effective specific activity is expected using this method. This method appears to be a useful alternative for the synthesis of various bromine-labelled radioligands for in vivo evaluation.
Applications of nucleophilic radiobromination using diaryl iodonium salt precursors
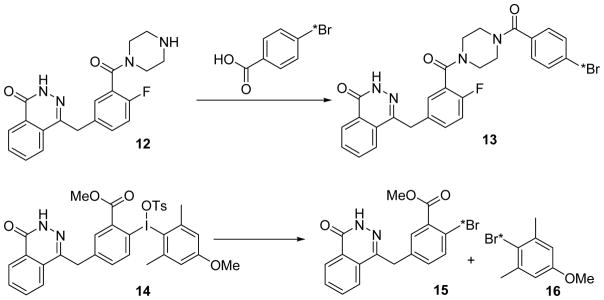
We have prepared a radiobromine-labelled olaparib derivative (13) by conjugating the amine precursor (12) with 4-[*Br]bromobenzoic acid; this compound is now available for evaluation as a radiotracer for poly ADP ribose polymerase-1 (PARP-1), a critical target for cancer therapy.21,22 The direct labeling using electrophilic destannylation of a tributyltin precursor may not work, based on reports on the synthesis of the corresponding radioiodine-labelled olaparib derivative.23.24 The method reported here has allowed us to radiosynthesize 13 for a preliminary evaluation simply under the conventional conjugation reaction conditions. The radiosynthesis of compound 13, which still needs optimization, is in the supporting information.
We also tested the base-free method on compound 14, which was synthesized for the purpose of 18F labeling of olaparib. Under microwave irradiation in DMSO, 2.3 mg of precursor (14) afforded over 96% incorporation of activity, 40% of which was compound 15 and 56% compound 16 as the other only radioactive product (see supporting information). Under the same conditions, as well as the conventional conditions, however, no18F incorporation was observed (unpublished work).
Conclusion
We have demonstrated the applicability of aromatic radiobromination by nucleophilic substitution using diaryliodonium salt precursors in model compounds. We have developed a QMA-treatment method and a base-free method that operate well even in partially aqueous solutions under conventional heating or microwave irradiation in good to high yields, and we have used these methods for the radiosynthesis of 4-bromobenzaldehyde and 4-bromobenzoate, which are building blocks for the preparation of radiobromopharmaceuticals. These methods will provide a convenient route for radiobromination of electron-deficient arenes and an alternative route for the synthesis of bromo-radiopharmaceuticals for biological evaluations.
Supplementary Material
Scheme 3.
Acknowledgments
Support from the following research grants is gratefully acknowledged: The Department of Energy (DOE: SC0008432) and the National Institutes of Health (NIH: CA025836, to J.A.K.). Some of the radioactivity was provided free of charge from the Cyclotron Facility in Washington University in Saint Louis. We also would like to thank the reviewers of this paper for the suggestion that resulted in the QMA method of direct activity elution.
References
- 1.Maziere B, Loch C. Radiopharmaceuticals Labeled with Bromine Isotopes. Appl Radiat Isot. 1986;37:703–713. doi: 10.1016/0883-2889(86)90264-9. [DOI] [PubMed] [Google Scholar]
- 2.Rowland DJ, McCarthy TJ, Welch MJ. Handbook of Radiopharmaceuticals. John Wiley & Sons, Ltd; 2005. Radiobromine for Imaging and Therapy; pp. 441–465. [Google Scholar]
- 3.Buchegger F, Perillo-Adamer F, Dupertuis YM, Delaloye AB. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol imaging. 2006;33:1352–1363. doi: 10.1007/s00259-006-0187-2. [DOI] [PubMed] [Google Scholar]
- 4.Tang L. Radionuclide production and yields at Washington University School of Medicine. Q J Nucl Med Mol Imaging. 2008;52:121–133. [PubMed] [Google Scholar]
- 5.Kassis AI, Adelstein SJ, Haydock C, Sastry KS, McElvany KD, Welch MJ. Lethality of Auger electrons from the decay of bromine-77 in the DNA of mammalian cells. Radiat Res. 1982;90:362–373. [PubMed] [Google Scholar]
- 6.Zhou D, Zhou H, Jenks CC, Lewis JS, Katzenellenbogen JA, Welch MJ. Bromination from the macroscopic level to the tracer radiochemical level: (76)Br radiolabeling of aromatic compounds via electrophilic substitution. Bioconjugate Chem. 2009;20:808–816. doi: 10.1021/bc800313c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pike VW, Aigbirhio F. Reactions of cyclotron-produced [18F]fluoride with diaryliodonium salts—a novel single-step route to no-carrier-added [18F]fluoroarenes. J Chem Soc Chem Commun. 1995;21:2215–2216. [Google Scholar]
- 8.Chun J-H, Pike VW. Single-step syntheses of no-carrier-added functionalized [18F] fluoroarenes as labeling synthons from diaryliodonium salts. Org Biomol Chem. 2013;11:6300–6306. doi: 10.1039/c3ob41353e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross TL, Ermert J, Hocke C, Coenen HH. Nucleophilic F-18-Fluorination of heteroaromatic iodonium salts with no-carrier-added [F-18]Fluoride. J Am Chem Soc. 2007;129:8018–8025. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]
- 10.Zhang MR, Kumata K, Takei M, Fukumura T, Suzuki K. How to introduce radioactive chlorine into a benzene ring using [*Cl]Cl-? Appl. Radiat Isot. 2008;66:1341–1345. doi: 10.1016/j.apradiso.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 11.McCarron JA, Dowsett K, Pike VW. Reactions of diaryliodonium salts with no-carrier-added [77Br]bromide and [11C]cyanide. XIIth Int. Symp. Radiopharm. Chem; 1997; Uppsala, Sweden. Jun 15–19, pp. 109–110. [Google Scholar]
- 12.Dimagno S, Hu B. WO2015147950 (A2) Radioiodinated compounds. 2015
- 13.Guérard F, Lee Y-S, Baidoo K, Gestin J-F, Brechbiel MW. Unexpected Behavior of the Heaviest Halogen Astatine in the Nucleophilic Substitution of Aryliodonium Salts. Chem Eur J. 2016;22:12332–12339. doi: 10.1002/chem.201600922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pike VW, Butt F, Shah A, Widdowson DA. Facile synthesis of substituted diaryliodonium tosylates by treatment of aryltributylstannanes with Koser’s reagent. J Chem Soc Perkin Trans I. 1999:245–248. [Google Scholar]
- 15.Bielawski M, Zhu M, Olofsson B. Efficient and General One-Pot Synthesis of Diaryliodonium Triflates: Optimization, Scope and Limitations. Adv Synth Catal. 2007;349:2610–2618. [Google Scholar]
- 16.Richarz R, Krapf P, Zarrad F, Urusova EA, Neumaier B, Zlatopolskiy BD. Neither azeotropic drying, nor base nor other additives: a minimalist approach to 18F-labeling. Org. Biomol. Chem. 2014;12:8094–8099. doi: 10.1039/c4ob01336k. [DOI] [PubMed] [Google Scholar]
- 17.Kasahara T, Jang YJ, Racicot L, Panagopoulos D, Liang SH, Ciufolini MA. Iodonium Metathesis Reactions. Angew Chem Int Edit. 2014;53:9637–9639. doi: 10.1002/anie.201405594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elander N, Jones JR, Lu SY, Stone-Elander S. Microwave-enhanced radiochemistry. Chem Soc Rev. 2000;29:239–2349. [Google Scholar]
- 19.Chun JH, Telu S, Lu SY, Pike VW. Radiofluorination of diaryliodonium tosylates under aqueous-organic and cryptand-free conditions. Org Biomol Chem. 2013;11:5094–5099. doi: 10.1039/c3ob40742j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wren JC. Radioiodine Chemistry: The Unfinished Story. Proceedings of the European Review Meeting on Severe Accident Research - ERMSAR; 2005; France. 2005. p. 438. [Google Scholar]
- 21.Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3:257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: A new era of targeted therapy. Maturitas. 2015;81:5–9. doi: 10.1016/j.maturitas.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Salinas B, Irwin CP, Kossatz S, Bolaender A, Chiosis G, Pillarsetty N, Weber WA, Reiner T. Radioiodinated PARP1 tracers for glioblastoma imaging. EJNMMI Res. 2015;5:123. doi: 10.1186/s13550-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zmuda F, Malviya G, Blair A, Boyd M, Chalmers AJ, Sutherland A, Pimlott SL. Synthesis and Evaluation of a Radioiodinated Tracer with Specificity for Poly(ADP-ribose) Polymerase-1 (PARP-1) in Vivo. J Med Chem. 2015;58:8683–8693. doi: 10.1021/acs.jmedchem.5b01324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.