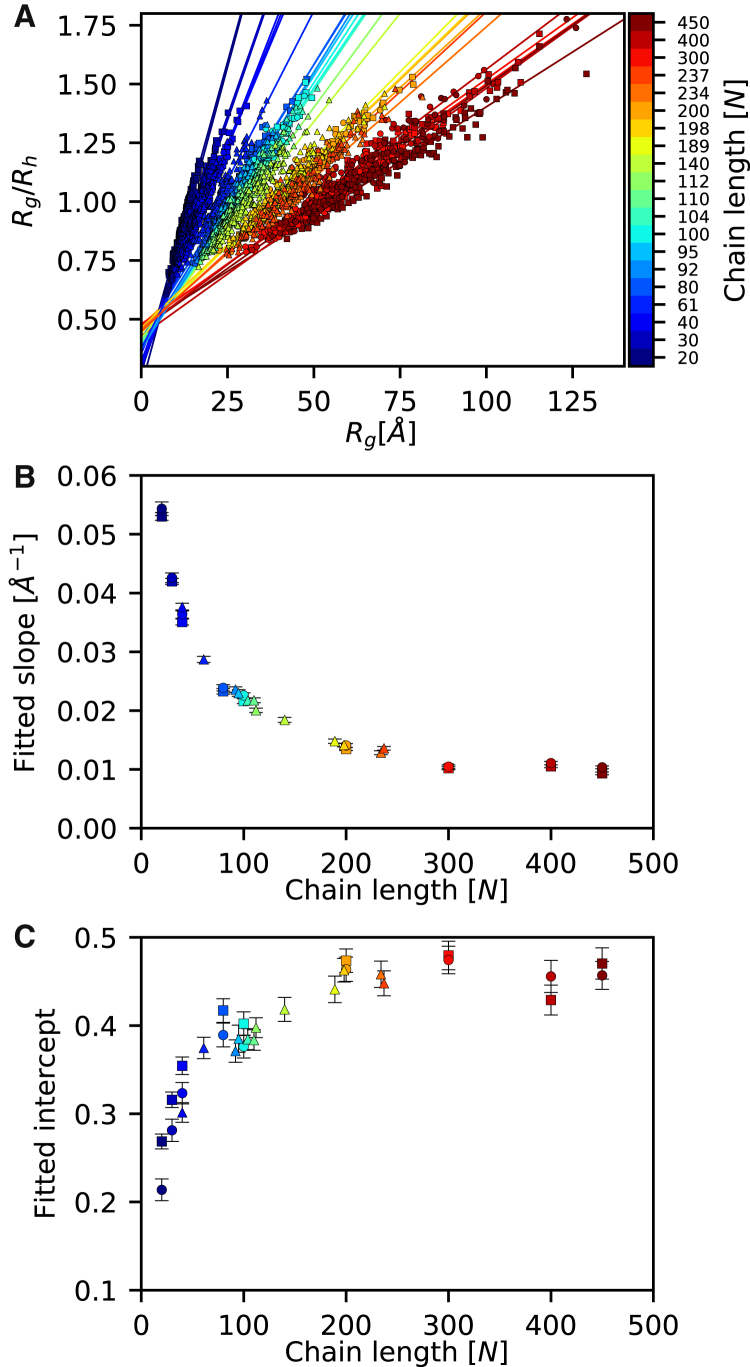
Figure 2.
An empirical relationship between Rg and Rh. For each of 30 polypeptides varying in length between 20 and 450 residues, we sampled 100 structures and calculated the hydrodynamic radius (Rh) and radius of gyration (Rg) for each structure. (A) In line with previous findings, we observed an approximately linear relationship between the ratio Rg/Rh and Rg, but with slope and intercept differing between polypeptides of different lengths (indicated by different colors). We fitted each dataset (indicated by the different shapes: squares for poly-valine; circles for IDP-like; and triangles for IDPs) to a straight line and observed that both the slope (B) and the intercept (C) systematically depended on the number of amino acid residues in the polypeptide. Error bars represent the error of fits. Note that the different sets of peptides appear to follow the same trends, suggesting that in this model it is the length of the peptide, not the composition, that is most relevant. The data for each peptide are shown separately in Figs. S1, S2, and S3. To see this figure in color, go online.