Figure 1. A proposed versatile approach to access proteins with lysine acylations.
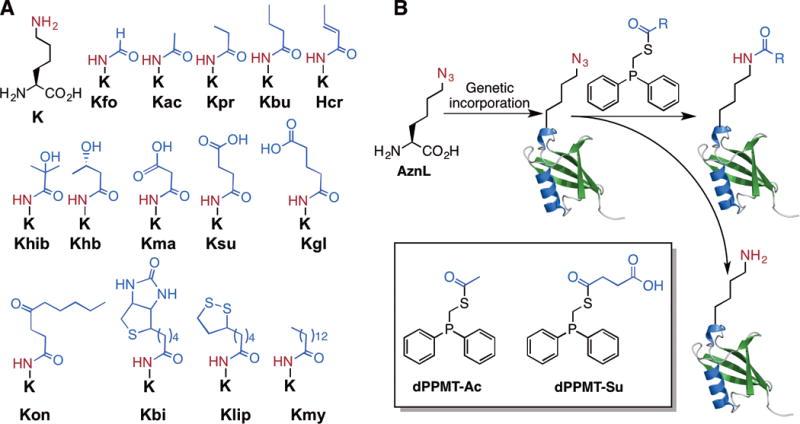
(A) Posttranslationally acylated lysines and their abbreviations. Acylations with small proteins such as ubiquitin and ubiquitin like proteins are not shown. (B) The genetic incorporation of AznL followed by traceless Staudinger ligation with a phosphinothioester to install a site-specific lysine acylation in a protein. The reducing nature of the phosphinothioester leads to the partial conversion of AznL to lysine as a side product. Shown in the inlet are two initially designed phosphinothioesters.
