Figure 1. MLK3 mediates lipotoxic stress-induced STAT1 phosphorylation via a MAPK signaling cascade in hepatocytes.
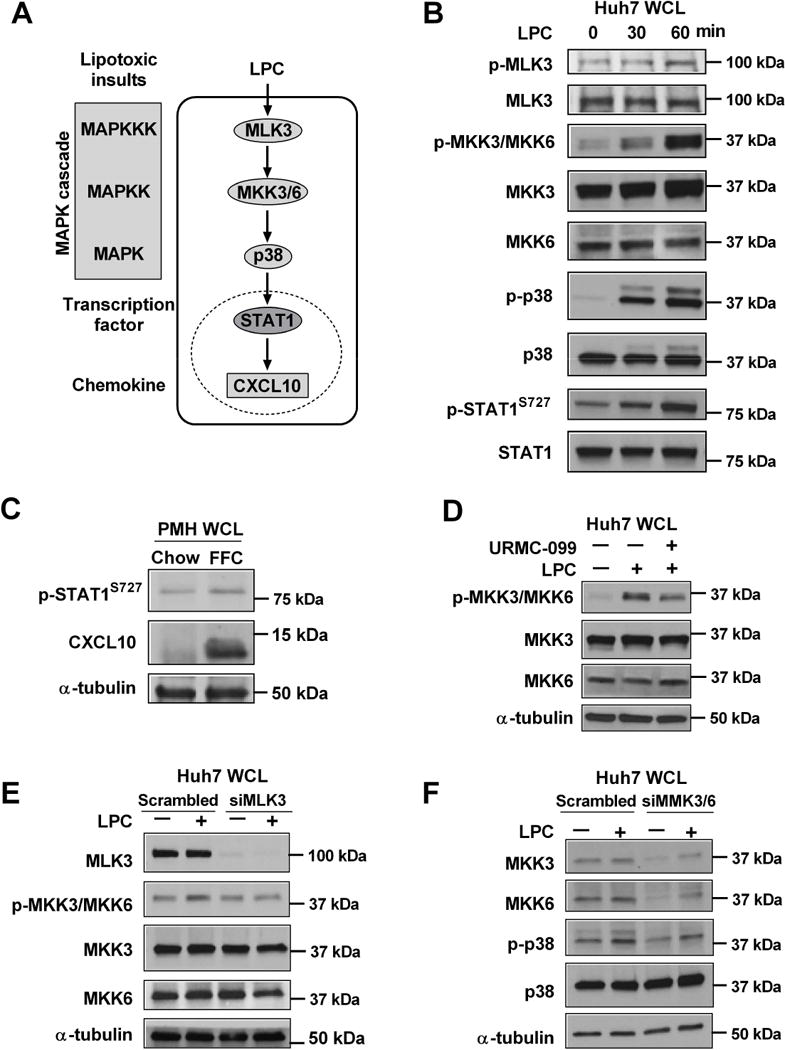
(A) Schematic representation of CXCL10 induction under lipotoxic conditions. MLK3, a mitogen activated protein kinase kinase kinase (MAPKKK), mediates CXCL10 induction by triggering a MAPK signaling pathway consisting of the mitogen activated protein kinase kinase (MAPKK) MKK3/6, and the mitogen activated protein kinase (MAPK) p38, which in turn induces STAT1 phosphorylation and transcriptional activation with subsequent CXCL10 induction. Immunoblot was used to assess: (B) Phosphorylated MLK3, MKK3/MKK6, p38, and STAT1 Ser727 and their respective total protein levels in whole cell lysates (WCL) from Huh7 cells treated with 40 μM LPC; (C) Phospho-STAT1 Ser727 and CXCL10 protein levels in primary mouse hepatocytes (PMH) isolated from high fat, fructose and cholesterol (FFC) or chow fed mice; (D) Phospho-MKK3/MKK6, total MKK3/MKK6 and α-tubulin levels on whole cell lysates from Huh7 cells treated with either vehicle, or 40 μM LPC for 1hr with and without 1 μM URMC-099 (URMC); (E) Phospho MKK3/6, total MLK3, MKK3, MKK6 and α-tubulin on whole cell lysates from Huh7 cells transfected with siMLK3 or scrambled siRNA and treated with vehicle or 40 μM LPC for 1 hr.; (F) Phospho-p38, total p38, total MKK3, MKK6 and α-tubulin in whole cell lysates from Huh7 cells transfected with siMKK3/6 or scrambled siRNA and treated with vehicle or 40 μM LPC for 1 hr.
