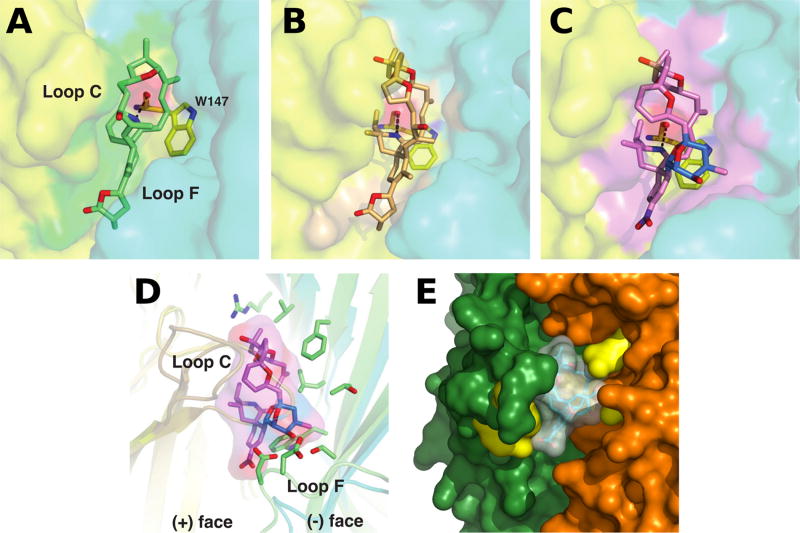
Fig. 2.
Close-up views of the binding interfaces of several cyclic imine toxins. Crystal structures of the AChBP subunit interface (the (+) and (−) faces are displayed in yellow and cyan, respectively) with bound gymnodimine A (A), 13-desmethyl spirolide C (B) and pinnatoxin A (C) (Protein Data Bank accession codes 2X00, 2WZY and 4XHE, Bourne et al. 2010, 2015); (D) Overlay of the binding interfaces of AChBP with bound pinnatoxin A and of the human α4β2 receptor (5KXI, Morales-Perez et al. 2016) (the α and β subunits are displayed in light orange and green, respectively). Those side chains from the β2 subunit ((−) face) that could contribute to pinnatoxin A binding are shown in green; (E) Docking complex of pinnatoxin A with the α7 nAChR structure generated by homology modeling (Aráoz et al. 2011). The α7 residues Trp147, Arg186, Tyr188, Tyr195 (in the (+) subunit) and Gln57, Gln116 (in the (−) subunit), which are predicted to establish hydrogen bonds with the toxin, are displayed in yellow.