Abstract
Mutations of the DJ-1 (PARK7) gene are linked to familial Parkinson's disease. We used gene targeting to generate DJ-1-deficient mice that were viable, fertile, and showed no gross anatomical or neuronal abnormalities. Dopaminergic neuron numbers in the substantia nigra and fiber densities and dopamine levels in the striatum were normal. However, DJ-1–/– mice showed hypolocomotion when subjected to amphetamine challenge and increased striatal denervation and dopaminergic neuron loss induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine. DJ-1–/–embryonic cortical neurons showed increased sensitivity to oxidative, but not nonoxidative, insults. Restoration of DJ-1 expression to DJ-1–/– mice or cells via adenoviral vector delivery mitigated all phenotypes. WT mice that received adenoviral delivery of DJ-1 resisted 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine-induced striatal damage, and neurons overexpressing DJ-1 were protected from oxidative stress in vitro. Thus, DJ-1 protects against neuronal oxidative stress, and loss of DJ-1 may lead to Parkinson's disease by conferring hypersensitivity to dopaminergic insults.
Parkinson's disease (PD) is a neurodegenerative disorder characterized by tremor, rigidity, akinesia, and postural instability (1). The cause of PD remains unknown, but epidemiological and genetic studies have suggested that the observed loss of dopaminergic neurons in PD is due to defects in common intracellular signaling pathways (2). Genes linked to familial PD include α-synuclein (3), Parkin (4), UCH-L1 (5), PINK1 (6), and dardarin (7). Proteins encoded by these genes are thought to be involved in protein aggregation and proteasome function, processes which, when disrupted in model systems, can also result in noninherited forms of PD (8). Recently, loss-of-function mutations in the DJ-1 locus were found in families with autosomal recessive early-onset PD (9). Additional studies have confirmed other DJ-1 mutations in various PD cohorts (10). DJ-1 was initially cloned as a putative oncogene (11) and as part of an RNA-binding complex (12). DJ-1 is highly expressed by normal astrocytes (13) and has been implicated in fertilization (14) and tumorigenesis (15, 16). Studies of the crystal structure of DJ-1 (17) suggest that a particular DJ-1 mutation (L166P) reduces DJ-1 protein stability (18–20), resulting in degradation through the ubiquitin–proteasome system (21, 22). However, the physiological function of DJ-1 remains largely unknown.
Motor impairments in PD patients result from inhibition of the nigrostriatal motor pathway. This inhibition is due to the loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) (8). The cause of the dopaminergic neuron loss remains unknown, but oxidative stress leading to apoptotic neuronal death has been implicated (23). Various neurotoxic paradigms have been studied in an effort to reproduce oxidative stress leading to neuronal loss in the SNc. Of these, administration of the well characterized meperidine analogue 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) results in pathology most similar to PD (24). When taken up by dopaminergic neurons, MPP+ (the active metabolite of MPTP) inhibits mitochondrial complex I and thus impairs respiration, leading to superoxide formation (23). Although in vitro studies have suggested that DJ-1 can protect cultured neuronal cell lines against the effects of oxidative stress (25), the in vivo role of DJ-1 has yet to be determined. To investigate the physiological function of DJ-1 and to examine the effect of DJ-1 deficiency in vivo, we have characterized gene-targeted DJ-1 knockout (DJ-1–/–) mice. We demonstrate that loss of DJ-1 exacerbates oxidative stress-induced cell death in primary cortical and dopaminergic neurons. Overexpression of DJ-1 can rescue these effects and protect WT neurons from oxidative stress. We also show that susceptibility to MPTP-induced striatal fiber and nigral neuronal loss is increased in DJ-1–/– mice. In WT mice, adenoviral-mediated overexpression of DJ-1 blocks MPTP-induced neuronal loss and protects the animals against neurodegeneration in the SNc. Our results point to a physiological role for DJ-1 in the protection of neurons against oxidative stress and environmental neurotoxins.
Experimental Procedures
Generation and Genotyping of DJ-1-Deficient Mice. The genomic murine DJ-1 gene was isolated from a 129/Sv library and used to generate a targeting construct in which DJ-1 exons 3–5 were replaced with a neomycin (Neo) selectable cassette. The first coding exon of DJ-1 was modified to contain a premature stop codon, resulting in a transcript corresponding to the first eight amino acids of the DJ-1 cDNA. Targeting constructs were electroporated into E14K embryonic stem cells (129/Ola). G418-resistant colonies were screened by PCR (forward primer, TGC TGA AAC TCT GCC ATG TGA ACC; reverse primer, CCT GCT TGC CGA ATA TCA T). PCR-positive colonies were confirmed by Southern blotting of EcoRI-digested genomic DNA by using flanking DJ-1 genomic and neomycin-specific probes. Successful homologous recombinants were injected into day 3.5 C57BL/6 blastocytes to generate chimeric mice. Chimeric males were crossed with C57BL/6 females to achieve germ-line transmission identified by coat color and confirmed by Southern blotting of tail genomic DNA. F1 progeny were backcrossed for seven generations to C57BL/6 mice, and heterozygotes were intercrossed to generate mice homozygous for the targeted DJ-1 allele. Genotypes of animals were verified by using PCR (WT DJ-1 forward primer, TGC TGA AAC TCT GCC ATG TGA ACC; WT DJ-1 reverse primer, CCT GCT TGC CGA ATA TCA T; and Neo, AGG TGA CAC TGC CAG TTG CTA GTC). PCR conditions were 95°C for 30 sec, 64°C for 30 sec, and 72°C for 1 min (40 cycles).
Generation of Anti-DJ-1 Antibody. Rabbit antiserum was raised against purified Trx-DJ-1 protein (Antibodies, Inc., Davis, CA) and purified by preadsorption on Trx-coupled CNBr-Sepharose 4B followed by affinity purification on GST-DJ-1 fusion protein coupled to CNBr-Sepharose 4B. Low-affinity antibody was eluted in a pH 5.0 buffer and stored. The high-affinity anti-DJ-1 antibody used in this study was eluted from the affinity column with 0.1 M glycine (pH 2.5).
Generation of DJ-1 Adenoviruses. Adenovirus vectors expressing DJ-1 were generated by subcloning WT DJ-1 cDNA or L166P mutant DJ-1 cDNA into pAdTRACK-CMV (26) in which the expression of GFP and DJ-1 is driven by separate cytomegalovirus promoters. Adenovirus was produced and titered as described (26).
Neuronal Cultures and Stimuli. Cortical neurons were cultured as described (27) from day 14–15 mouse embryos either of the CD1 strain (Charles River Laboratories) or from DJ-1+/+, DJ-1+/–, or DJ-1–/–animals generated by knockout breeding. On day 1–2 after the initial plating, neurons were cultured in serum-free medium supplemented with H2O2 (30 μM), camptothecin (10 μM), or staurosporine (2 μM). Numbers of viable neurons were evaluated by lysis of cultures followed by the counting of intact nuclei as described (27).
For overexpression studies involving H2O2 treatment, cortical neurons were exposed to recombinant adenovirus expressing GFP only, GFP plus DJ-1 cDNA, or GFP plus DJ-1 L166P mutant cDNA, either at the time of plating or 24 h after plating (multiplicity of infection, 100). At 24–36 h postinfection, cultures were incubated with 30 μM H2O2 and then fixed. Infected cells were identified by GFP fluorescence, and nuclear integrity was assessed by Hoechst staining as described (28). Percent neuronal survival was calculated as the percentage of live GFP-positive neurons over the total number of neurons expressing GFP.
For experiments involving mesencephalic neurons, midbrain cultures were harvested from day 13–14 embryos as described (29). Cells were incubated with anti-tyrosine hydroxylase (TH) antibody (ImmunoStar, Hudson, WI; 1:10,000) as the primary antibody and Cy3-linked anti-mouse antibody (1:300, The Jackson Laboratory) as the secondary antibody. Nuclei were stained with Hoechst dye. TH-positive (TH+) cells were visualized by fluorescence microscopy, and neuronal viability was evaluated by nuclear morphology. Viability was expressed as the percentage of viable TH+ neurons in the treated culture compared with untreated controls.
MPTP Treatment and Adenoviral Gene Delivery in Vivo. Littermate male mice (8–10 wk old) of the C57BL/6 background were used for all MPTP experiments. On 5 consecutive days, mice received i.p. injections of either MPTP/HCl (Sigma; 25 mg of free base per kg of body weight per day) or an equivalent volume of 0.9% saline. At 14 days post-MPTP treatment, all mice were killed and their neurons analyzed. For mice pretreated with adenoviral vectors, viruses were injected unilaterally into the right striatum as described (30). The adenovirus was delivered into the striatum 7 days before the initiation of MPTP treatment. All animal experiments conformed to the guidelines set by the Canadian Council for the Use and Care of Animals in Research and the Canadian Institutes of Health Research and were conducted with the approval of the University of Ottawa and Ontario Cancer Institute Animal Care Committees.
Immunohistochemistry. Mice were killed and brains harvested as described (31). Free-floating sections were incubated with primary anti-TH antibody (1:10,000, ImmunoStar), anti-DJ-1 antibody (1:1,000), a biotinylated secondary antibody (1:200, Jackson ImmunoResearch), and a streptavidin horseradish peroxidase tertiary antibody (1:200, Amersham Pharmacia Bioscience).
Neuronal Loss Assessment. After immunohistochemical analysis, TH+ neurons were counted as described (32). Loss of TH+ or cresyl violet-stained cells in the nigral region was used as an index of dopaminergic cell loss after MPTP treatment. For adenoviral experiments, TH+ cell counts were performed as described (30) at the level of medial terminal nucleus, the region in which adenovirus-mediated gene expression is highest (30). For analyses of TH and dopamine transporter protein (DAT) intensity in the striatum, sections were analyzed by densitometry by using northern eclipse image software, as described (30).
HPLC and MPP+ Analysis. Analyses of dopamine, dopamine metabolites, and MPP+ were carried out as described (33).
Behavioral Analyses. Novel environment. Walled 30 × 30-inch square arenas were used for open field testing of mice by using a video camera and analysis software, as described (30). Motor impairments. Assessment of the ability of mice to descend and cross poles (pole test) was carried out as described (34). Somatosensory. The adhesive removal test was used to assess somatosensory ability and was carried out as described (35). Home cage. Animal behavior was assessed by using a home-cage mouse-monitoring system that utilizes the beam-break sensor apparatus (MicroMax, Accuscan, Columbus, OH). Animals were monitored for 24 h before MPTP treatment or starting on day 13 after the initiation of MPTP treatment. To assess “dopamine-related” behavioral output, mice were monitored for 1 hr after amphetamine challenge (2 mg/kg) (30).
Results
Generation of DJ-1 Knockout Mice. We generated DJ-1-deficient mice by gene-targeting (Fig. 6A, which is published as supporting information on the PNAS web site) and confirmed disruption of the DJ-1 locus by Southern blotting (Fig. 6B). Northern blotting of RNA from DJ-1–/–mouse embryonic fibroblasts (Fig. 6C) and Western blotting of extracts of DJ-1–/–cortical neurons (Fig. 6D) confirmed the lack of endogenous DJ-1 RNA and protein expression. DJ-1+/– and DJ-1–/– mice were born at the expected Mendelian frequencies, were viable and fertile, and showed no gross anatomical or neuronal abnormalities.
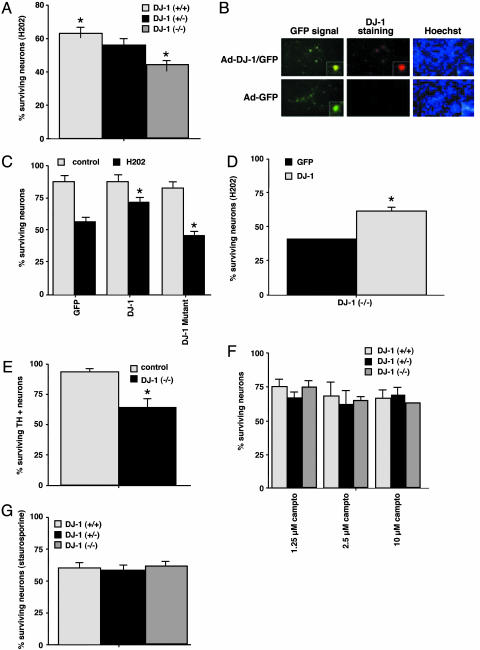
DJ-1 Protects Primary Neurons Against Cell Death Induced by Oxidative Stress. To determine whether DJ-1 could protect primary neuronal cells against oxidative stress, we subjected primary cortical neurons derived from the brains of DJ-1+/+, DJ-1+/–, and DJ-1–/–embryos to oxidative stress in the form of H2O2. DJ-1–/–neurons showed a 20% increase in cell death compared with DJ-1+/+ neurons (Fig. 1A). An intermediate amount of cell death was observed in DJ-1+/–neurons, suggesting a gene-dosage effect. To determine whether overexpression of DJ-1 could protect primary DJ-1+/+ neurons from oxidative death, we constructed adenovirus vectors expressing either GFP alone (control) or GFP plus WT DJ-1 (Fig. 1B). Primary DJ-1+/+ cortical neurons were infected with these vectors and treated with H2O2. Overexpression of WT DJ-1 protected DJ-1+/+ neurons against H2O2-induced apoptosis (Fig. 1C). However, this protection was not observed if DJ-1+/+ neurons were infected with an adenovirus vector expressing GFP alone or GFP plus the mutated L166P DJ-1 protein (Fig. 1C). Indeed, a slight increase in cell death was observed in neurons expressing L166P DJ-1, suggesting that this mutant protein may act as a dominant negative inhibitor of WT DJ-1. When we overexpressed WT DJ-1 in DJ-1–/–cortical neurons, the hypersensitivity of the mutant cells to H2O2 was reduced (Fig. 1D), confirming that DJ-1 protects primary neurons from oxidative stress.
Fig. 1.
DJ-1 protects against cell death induced by oxidative, but not nonoxidative, stress. Survival determinations for each of the following experiments are described in Experimental Procedures. (A) Increased sensitivity to H2O2 in the absence of DJ-1. Cortical neurons from DJ-1+/+, DJ-1+/–, and DJ-1–/–embryos were treated for 3 h with 30 μM H2O2. (B) Immunofluorescence analysis of DJ-1+/+ embryonic cortical neurons infected with an adenoviral vector (Ad) expressing either GFP or GFP plus DJ-1. Hoechst nuclear staining of the same culture is also shown. (C) Protective effect of DJ-1 overexpression in DJ-1+/+ cortical neurons. DJ-1+/+ neurons infected with adenovirus expressing GFP plus WT DJ-1 protein, but not GFP plus mutated (L166P) DJ-1 protein, showed increased survival after H2O2 exposure. Control, no H2O2. (D) Protective effect of WT DJ-1 in DJ-1–/–cortical neurons. DJ-1–/–neurons infected with adenovirus expressing GFP plus WT DJ-1 showed increased survival after H2O2 exposure. (E) Increased sensitivity of DJ-1–/–mesencephalic dopaminergic neurons (TH+) to other oxidative insults. DJ-1–/–cortical neurons were exposed to 10 nM rotenone for 24 h. (F and G) Lack of sensitivity of DJ-1–/–cortical neurons (TH+) to nonoxidative insults. The indicated doses of camptothecin (Fig. 1F) or 2 μM staurosporine (Fig. 1G) were applied to DJ-1+/+, DJ-1+/–, and DJ-1–/–neurons for 16 h. For A and C–G, each data point is the mean ± SEM of three to five independent cultures, where * denotes a significance level of P < 0.05.
The pesticide rotenone is another oxidative stressor thought to promote neuronal death in PD (36). Rotenone inhibits mitochondrial complex I and increases reactive oxygen species (37). We treated DJ-1–/–mesencephalic dopaminergic neurons (which stain positively for TH+) with rotenone and analyzed cell death. The survival of rotenone-treated DJ-1–/–TH+ neurons was decreased by 30% compared with rotenone-treated DJ-1+/+ neurons (Fig. 1E).
To investigate whether DJ-1 protects against nonoxidative insults, cortical neurons from DJ-1+/+, DJ-1+/–, and DJ-1–/–embryos were treated with various doses of camptothecin, a topoisomerase I inhibitor. No statistically significant differences in cell death were observed among the genotypes (Fig. 1F). Neither were DJ-1–/–neurons more susceptible than controls to treatment with the protein kinase inhibitor staurosporine (Fig. 1G).
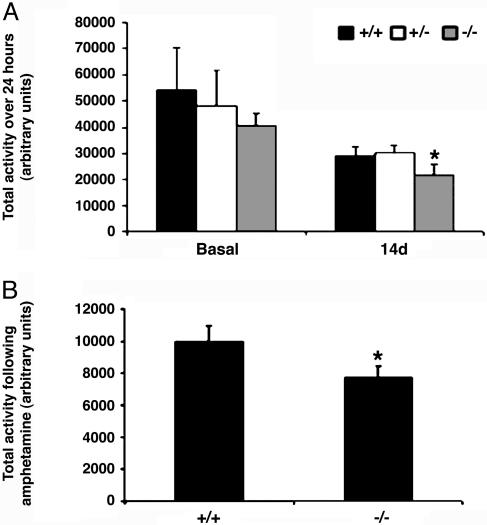
Behavioral Defects in DJ-1–/–Mice. To determine whether DJ-1–/– mice exhibited gross motor behavior abnormalities resembling those in PD patients, we carried out open field locomotion analyses over a 24-h period using automated beam break analyses in a home-cage environment. However, no statistically significant differences in spontaneous locomotion could be detected among DJ-1+/+, DJ-1+/–, and DJ-1–/– mice (8 weeks old; C57BL/6) (Fig. 2A Left), a result confirmed in aged mice (13 months) of mixed background (data not shown). Additional behavior analyses, including the pole (34), novel environment (30), and somatosensory tests (35), were performed on aged DJ-1–/– mice, but no anomalies were observed (data not shown). Because C57BL/6 DJ-1–/– mice tended to display a slightly lower average locomotor activity than controls, we challenged C57BL/6 DJ-1–/– mice with MPTP. The locomotor activity of MPTP-treated DJ-1+/+ and DJ-1+/– mice was reduced by 50% compared with saline-treated controls (Fig. 2A Right). However, compared with MPTP-treated DJ-1+/+ mice, MPTP-treated DJ-1–/– mice showed a statistically significant decrease in total activity (Fig. 2A Right). In a parallel experiment, we challenged DJ-1+/+ and DJ-1–/– mice with amphetamine, which leads to dopamine release (38) and hyperlocomotion in WT mice (39). DJ-1–/– mice exhibited a significant depression in amphetamine-induced locomotor activity compared with controls (Fig. 2B). Thus, although unchallenged locomotor behavior is normal in the absence of DJ-1, a mild deficit exists that is revealed upon challenge of the dopaminergic system.
Fig. 2.
Minor behavioral defects are revealed in DJ-1–/– mice only upon MPTP or amphetamine challenge. (A) Open-field locomoter activity measured over a 24-h period in either unchallenged animals (basal) or animals that had been challenged 14 days earlier with MPTP (30 mg/kg per day for 5 days) (n = 6–10 animals per group). (B) Open-field activity measured for 1 h in DJ-1+/+ and DJ-1–/– mice (n = 4 animals/group) challenged with 2 mg/kg amphetamine. Total activity was measured in arbitrary units and plotted as the mean ± SEM, where * denotes a significance level of P < 0.05.
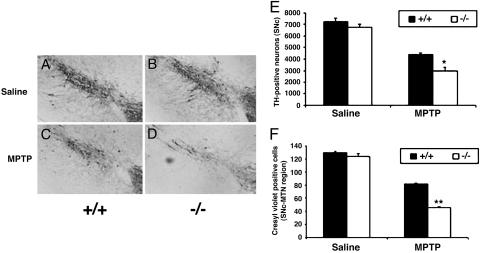
Loss of DJ-1 Confers Susceptibility to MPTP-Induced Neuronal Death. We next histologically examined the effects of chronic MPTP neurotoxicity on DJ-1–/– mice. Saline-treated DJ-1+/+ and DJ-1–/– mice showed no significant differences in TH+ neurons (Fig. 3 A and B). However, at 14 days post-MPTP treatment, immunostaining of DJ-1+/+ SNc sections showed decreased TH+ neuron numbers compared with saline-treated controls (Fig. 3 A and C). Strikingly, DJ-1–/– mice showed a much greater relative decrease in the number of viable TH+ SNc neurons after MPTP treatment (Fig. 3 B and D), suggesting that loss of DJ-1 sensitizes SNc neurons to MPTP-induced apoptosis. Quantitation of TH+ neuron numbers in MPTP-treated DJ-1+/+ and DJ-1–/– mice confirmed the histological findings (Fig. 3E). Similar results were obtained by using cresyl violet staining (40) as an independent marker of neuronal survival (Fig. 3F).
Fig. 3.
Loss of DJ-1 confers susceptibility to MPTP-induced neuronal death. (A–D) DJ-1+/– and DJ-1–/– mice were treated with MPTP or saline (control) as for Fig. 2, and sections of the SNc at the level of the medial terminal nucleus were prepared 14 days later. Sections were immunostained to detect TH+ neurons. Shown are the SNc of saline-treated DJ-1+/+ (A) and DJ-1–/–(B) mice and SNc of MPTP-treated DJ-1+/+ (C) and DJ-1–/–(D) mice. (E and F) Quantification of SNc neurons in the mice in A–D using either TH staining (E) or cresyl violet staining (F). Data in E and F are presented as mean ± SEM (n = 5–7 animals per group), where * and ** denote significance levels (ANOVA) of P < 0.05 and P < 0.01, respectively.
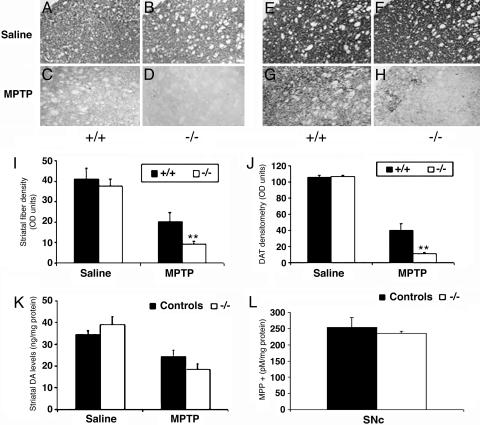
DJ-1–/– Mice Show Increased Striatal Denervation After MPTP Treatment. We confirmed that MPTP induces a greater loss of dopaminergic neurons in the absence of DJ-1 by examining dopaminergic terminal fiber density and dopamine levels in the striatal target region. Saline-treated DJ-1+/+ and DJ-1–/– mice showed similar TH staining in the striatum (Fig. 4 A and B). Although MPTP-treated DJ-1+/+ mice displayed the expected moderate depletion of dopaminergic fibers (Fig. 4 A and C), loss of DJ-1 exacerbated this effect (Fig. 4 B and D). Adjacent tissue sections immunostained for DAT revealed similar results (Fig. 4 E–H). This enhanced loss of striatal fiber density was confirmed by densitometric analysis of the TH-(Fig. 4I) and DAT-stained (Fig. 4J) sections. HPLC analyses revealed a significant reduction in striatal dopamine in all MPTP-treated mice compared with saline-treated controls (Fig. 4K). However, the relative loss of dopamine in MPTP-treated DJ-1–/– mice exceeded that in MPTP-treated DJ-1+/+ mice (Fig. 4K), consistent with our histological data. No significant differences in dopamine metabolites were detected between DJ-1+/+ and DJ-1–/– mice (data not shown). Importantly, the increased MPTP sensitivity of DJ-1–/– mice was not due to increased production of MPP+ in either the SNc (Fig. 4L) or the striatum (data not shown). Loss of DJ-1 thus increases susceptibility to dopaminergic neuron degeneration in vivo, which in turn leads to decreased striatal dopamine.
Fig. 4.
DJ-1–/– mice show increased striatal denervation after MPTP treatment. DJ-1+/+ and DJ-1–/– mice were treated with MPTP or saline as for Fig. 2, and striatal sections were prepared 14 days later. Sections were immunostained to detect either TH+ neurons (A–D) or DAT+ neurons (E–H). DJ-1+/+ mice were treated with saline (A and E) or MPTP (C and G), and DJ-1–/– mice were treated with saline (B and F) or MPTP (D and H). (I and J) Quantification of striatal fiber density (I; TH staining) and nerve density (J; DAT staining) of mice in A–H. HPLC determination of striatal dopamine (DA) levels (K) and nigral MPP+ levels (L) in DJ-1 control (+/+ or +/–) and DJ-1–/– mice. All animals were treated with either MPTP or saline as for Fig. 2 and evaluated on day 14. Data in I–L are presented as mean ± SEM (n = 4–8 animals/group), where * and ** denote significance levels (ANOVA) of P < 0.05 and P < 0.01, respectively.
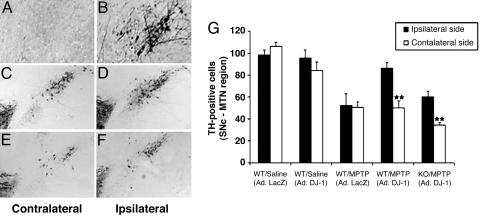
MPTP-Induced Neuronal Loss Is Rescued by Adenoviral Expression of DJ-1. To investigate whether DJ-1 could protect against oxidative insults in vivo, we engineered the restoration of DJ-1 expression in the SNc of DJ-1–/– mice using retrograde transportation of adenoviruses (31) expressing either DJ-1 or LacZ (control). Viral vectors were injected into one hemisphere of the striatum of DJ-1–/– mice. Virally introduced DJ-1 (but not LacZ) led to substantial DJ-1 expression in the SNc of the ipsilateral side of injection (Fig. 5 A and B).
Fig. 5.
MPTP-induced neuronal loss is rescued by adenoviral expression of DJ-1. (A and B) DJ-1–/– mice were injected unilaterally in the SNc with adenoviral vectors expressing either DJ-1 or LacZ (control). Sections of the SNc at the medial terminal nucleus were immunostained with anti-DJ-1 antibody to detect adenoviral-mediated DJ-1 expression. (A) DJ-1 expression is not visible in the contralateral (uninjected) hemisphere of DJ-1–/– SNc. (B) Upon injection of vector expressing WT DJ-1, DJ-1 expression is restored to the ipsilateral hemisphere of DJ-1–/– SNc. (C–F) DJ-1+/+ and DJ-1–/– mice were injected unilaterally in the SNc with adenoviral vectors expressing either DJ-1 or LacZ (control; not shown). Injected animals were then treated with MPTP as for Fig. 2. SNc sections were prepared and immunostained as for A and B above. The contralateral hemisphere served as the noninjected, MPTP-treated control. (C)TH+ neurons are reduced on the contralateral (no DJ-1 injection) side of the SNc of an MPTP-treated DJ-1+/+ mouse when compared with (D) the ipsilateral (DJ-1-injected) side of the same mouse (E). Very few TH+ neurons are present in contralateral side of the SNc from an MPTP-treated DJ-1–/– mouse, reflecting the hypersensitivity of these animals to oxidative stress. (F) Overexpression of DJ-1 in the ipsilateral side of the DJ-1–/– SNc attenuates this neuronal loss. (G) Quantification of numbers of TH+ neurons in the contralateral and ipsilateral SNc hemispheres of all animals in the groups represented in A–F. Data in G are presented as mean ± SEM (n = 4–5 animals per group), where ** denotes a significance level (ANOVA) of P < 0.01.
We then examined whether virally introduced DJ-1 could protect DJ-1+/+ and/or DJ-1–/– mice from MPTP-induced neurodegeneration. Viral vectors expressing either DJ-1 or LacZ were unilaterally injected into the striatum of DJ-1+/+ and DJ-1–/– mice 7 days before in vivo MPTP or saline (control) treatment. The contralateral side of the striatum of MPTP-treated mice served as the MPTP-treated uninjected control. Expression of DJ-1 in DJ-1+/+ (Fig. 5 C, D, and G), and DJ-1–/– (Fig. 5 E–G) mice prevented much of the neuronal loss induced by MPTP. In saline-treated DJ-1+/+ mice, expression of DJ-1 did not alter SNc neuron numbers compared with LacZ-expressing controls (Fig. 5G). As expected, MPTP treatment of LacZ-expressing DJ-1+/+ mice induced a 30–40% decrease in TH+ neuron numbers in both the ipsilateral and contralateral hemispheres (Fig. 5G). However, DJ-1 expression in the ipsilateral SNc of MPTP-treated DJ-1+/+ or DJ-1–/– mice resulted in a statistically significant rescue of TH+ neuron numbers (Fig. 5G). These data show that oxidative stress resulting from MPTP-mediated inhibition of mitochondrial complex I can be mitigated by overexpression of DJ-1. Thus, the hypersensitivity to MPTP observed in DJ-1–/– mice is a direct result of their DJ-1 deficiency.
Discussion
In this study, we characterized gene-targeted mice lacking DJ-1, a gene associated with familial PD. Mice deficient in DJ-1 are viable and fertile and produce viable offspring at the expected Mendelian frequencies. These data preclude a role for DJ-1 in embryogenesis or fertility. Rather, our results strongly suggest that a key physiological role of DJ-1 is to protect dopaminergic neurons in the SNc from oxidative stress. Although DJ-1 has been previously shown to protect cultured neuronal cell lines from oxidative stress in vitro, our results demonstrate this protective function in primary neurons in vivo. Furthermore, our data indicate that loss of DJ-1 function increases the sensitivity of primary neurons to oxidative stress and thus may promote neurodegeneration and PD development.
DJ-1 is highly expressed in neuronal and nonneuronal cells, and it has been proposed that DJ-1 might exert its neuroprotective effects by influencing the interaction of neurons and glia (13). However, in this study, we show that intrinsic loss of DJ-1 in primary neurons is sufficient to confer hypersensitivity to oxidative stress. Furthermore, these effects can be rescued by expression of the exogenous WT DJ-1 protein but not of the mutated L166P DJ-1 protein. Crystallographic studies of DJ-1 have shown that WT DJ-1 acts as a dimer, and that the L166P mutation inhibits dimer formation by breaking the C-terminal helix (17). When expressed in WT primary neurons, L166P DJ-1 may act as a dominant-negative inhibitor of DJ-1 dimerization such that the WT DJ-1 protein is no longer able to exert its antioxidative effect (41). In PD patients, L166P expression in neurons appears to represent a loss-of-function mutation (9). When we specifically examined the role of DJ-1 in dopaminegeric neurons, treatment of these cells with the environmental toxin rotenone revealed a heightened sensitivity in the absence of DJ-1. We also showed that DJ-1 deficiency does not increase susceptibility to neuronal death induced by nonoxidative stimuli, further supporting the hypothesis that neuronal death in PD patients is specifically due to oxidative stress.
DJ-1–/– mice do not display any gross neuronal abnormalities or motor deficits. Furthermore, untreated DJ-1–/– mice do not exhibit alterations in: (i) the number of TH+ neurons in the SNc, (ii) the density of TH fibers or levels of dopamine transporter in the target striatal region, or (iii) striatal dopamine levels. Only slight motor deficits are seen when mutant mice are treated with amphetamine. These findings indicate that loss of DJ-1 alone is not sufficient to produce PD symptoms. However, DJ-1–/– mice show a heightened tendency to develop PD-like pathology after MPTP treatment. MPTP reproduces PD pathology in the SNc of both humans and mice by affecting the oxidative balance in dopaminergic neurons (24). We found that the striatal denervation in MPTP-treated DJ-1–/– mice was highly reminiscent of the SNc neuronal loss observed in human PD patients. Future study of these mutant animals may reveal additional deficits and should allow an examination of how the dose of an oxidative agent affects susceptibility to neurodegeneration. Our DJ-1–/– mice thus represent a valuable model in which to examine the molecular mechanisms underlying PD.
Supplementary Material
Acknowledgments
We are grateful to Mary Saunders for scientific editing of the manuscript. D.S.P. was supported by the Canadian Institutes of Health Research (CIHR), the Parkinson's Society of Canada, the Parkinson's Disease Foundation, the Michael J. Fox Foundation, and the Canadian Stroke Network. D.S.P. is a CIHR scholar, and H.A. and S.H. are Canada Research Chairs in Neuroscience. R.H.K. and S.K.K. are recipients of M.D./Ph.D. scholarships from the CIHR. R.H.K. is also supported by the Frank Fletcher Memorial Fund, the David Rae Scholarship, and the Paul Starita Fellowship.
Author contributions: R.H.K., P.D.S., H. Aleyasin, S.H., S.P., S.K.K., D.W., A.M.L., D.S.P., and T.W.M. designed research; R.H.K., P.D.S., H. Aleyasin, S.H., M.P.M., A.W., A.J.Y.-T., and H. Anisman performed research; P.H. contributed new reagents/analytic tools; R.H.K. and D.S.P. analyzed data; and R.H.K. and D.S.P. wrote the paper.
Abbreviations: MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine; MPP+, active metabolite of MPTP; PD, Parkinson's disease; SNc, substantia nigra pars compacta; TH, tyrosine hydroxylase; DAT, dopamine transporter protein.
References
- 1.Lang, A. E. & Lozano, A. M. (1998) N. Engl. J. Med. 339, 1044–1053. [DOI] [PubMed] [Google Scholar]
- 2.Dawson, T. M. & Dawson, V. L. (2003) Science 302, 819–822. [DOI] [PubMed] [Google Scholar]
- 3.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997) Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- 4.Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y. & Shimizu, N. (1998) Nature 392, 605–608. [DOI] [PubMed] [Google Scholar]
- 5.Leroy, E., Boyer, R., Auburger, G., Leube, B., Ulm, G., Mezey, E., Harta, G., Brownstein, M. J., Jonnalagada, S., Chernova, T., et al. (1998) Nature 395, 451–452. [DOI] [PubMed] [Google Scholar]
- 6.Valente, E. M., Abou-Sleiman, P. M., Caputo, V., Muqit, M. M., Harvey, K., Gispert, S., Ali, Z., Del Turco, D., Bentivoglio, A. R., Healy, D. G., et al. (2004) Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- 7.Paisan-Ruiz, C., Jain, S., Evans, E. W., Gilks, W. P., Simon, J., van der Brug, M., de Munain, A. L., Aparicio, S., Gil, A. M., Khan, N., et al. (2004) Neuron 44, 595–600. [DOI] [PubMed] [Google Scholar]
- 8.Chung, K. K., Dawson, V. L. & Dawson, T. M. (2003) J. Neurol. 250, 15–24. [DOI] [PubMed] [Google Scholar]
- 9.Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., Dekker, M. C., Squitieri, F., Ibanez, P., Joosse, M., et al. (2003) Science 299, 256–259. [DOI] [PubMed] [Google Scholar]
- 10.Tan, E. K., Tan, C., Zhao, Y., Yew, K., Shen, H., Chandran, V. R., Teoh, M. L., Yih, Y., Pavanni, R., Wong, M. C., et al. (2004) Neurosci. Lett. 367, 109–112. [DOI] [PubMed] [Google Scholar]
- 11.Nagakubo, D., Taira, T., Kitaura, H., Ikeda, M., Tamai, K., Iguchi-Ariga, S. M. & Ariga, H. (1997) Biochem. Biophys. Res. Commun. 231, 509–513. [DOI] [PubMed] [Google Scholar]
- 12.Hod, Y., Pentyala, S. N., Whyard, T. C. & El-Maghrabi, M. R. (1999) J. Cell. Biochem. 72, 435–444. [PubMed] [Google Scholar]
- 13.Bandopadhyay, R., Kingsbury, A. E., Cookson, M. R., Reid, A. R., Evans, I. M., Hope, A. D., Pittman, A. M., Lashley, T., Canet-Aviles, R., Miller, D. W., et al. (2004) Brain 127, 420–430. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi, K., Taira, T., Niki, T., Seino, C., Iguchi-Ariga, S. M. & Ariga, H. (2001) J. Biol. Chem. 276, 37556–37563. [DOI] [PubMed] [Google Scholar]
- 15.Le Naour, F., Misek, D. E., Krause, M. C., Deneux, L., Giordano, T. J., Scholl, S. & Hanash, S. M. (2001) Clin. Cancer Res. 7, 3328–3335. [PubMed] [Google Scholar]
- 16.MacKeigan, J. P., Clements, C. M., Lich, J. D., Pope, R. M., Hod, Y. & Ting, J. P. (2003) Cancer Res. 63, 6928–6934. [PubMed] [Google Scholar]
- 17.Tao, X. & Tong, L. (2003) J. Biol. Chem. 278, 31372–31379. [DOI] [PubMed] [Google Scholar]
- 18.Olzmann, J. A., Brown, K., Wilkinson, K. D., Rees, H. D., Huai, Q., Ke, H., Levey, A. I., Li, L. & Chin, L. S. (2004) J. Biol. Chem. 279, 8506–8515. [DOI] [PubMed] [Google Scholar]
- 19.Moore, D. J., Zhang, L., Dawson, T. M. & Dawson, V. L. (2003) J. Neurochem. 87, 1558–1567. [DOI] [PubMed] [Google Scholar]
- 20.Macedo, M. G., Anar, B., Bronner, I. F., Cannella, M., Squitieri, F., Bonifati, V., Hoogeveen, A., Heutink, P. & Rizzu, P. (2003) Hum. Mol. Genet. 12, 2807–2816. [DOI] [PubMed] [Google Scholar]
- 21.Miller, D. W., Ahmad, R., Hague, S., Baptista, M. J., Canet-Aviles, R., McLendon, C., Carter, D. M., Zhu, P. P., Stadler, J., Chandran, J., et al. (2003) J. Biol. Chem. 278, 36588–36595. [DOI] [PubMed] [Google Scholar]
- 22.Gorner, K., Holtorf, E., Odoy, S., Nuscher, B., Yamamoto, A., Regula, J. T., Beyer, K., Haass, C. & Kahle, P. J. (2004) J. Biol. Chem. 279, 6943–6951. [DOI] [PubMed] [Google Scholar]
- 23.Jenner, P. (2003) Ann. Neurol. 53, S26–S38. [DOI] [PubMed] [Google Scholar]
- 24.Przedborski, S. & Vila, M. (2003) Ann. N.Y. Acad. Sci. 991, 189–198. [PubMed] [Google Scholar]
- 25.Taira, T., Saito, Y., Niki, T., Iguchi-Ariga, S. M., Takahashi, K. & Ariga, H. (2004) EMBO Rep. 5, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He, T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W. & Vogelstein, B. (1998) Proc. Natl. Acad. Sci. USA 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giovanni, A., Keramaris, E., Morris, E. J., Hou, S. T., O'Hare, M., Dyson, N., Robertson, G. S., Slack, R. S. & Park, D. S. (2000) J. Biol. Chem. 275, 11553–11560. [DOI] [PubMed] [Google Scholar]
- 28.Ghahremani, M. H., Keramaris, E., Shree, T., Xia, Z., Davis, R. J., Flavell, R., Slack, R. S. & Park, D. S. (2002) J. Biol. Chem. 277, 35586–35596. [DOI] [PubMed] [Google Scholar]
- 29.Cheung, N. S., Hickling, Y. M. & Beart, P. M. (1997) Neurosci. Lett. 233, 13–16. [DOI] [PubMed] [Google Scholar]
- 30.Crocker, S. J., Smith, P. D., Jackson-Lewis, V., Lamba, W. R., Hayley, S. P., Grimm, E., Callaghan, S. M., Slack, R. S., Melloni, E., Przedborski, S., et al. (2003) J. Neurosci. 23, 4081–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crocker, S. J., Lamba, W. R., Smith, P. D., Callaghan, S. M., Slack, R. S., Anisman, H. & Park, D. S. (2001) Proc. Natl. Acad. Sci. USA 98, 13385–13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, P. D., Crocker, S. J., Jackson-Lewis, V., Jordan-Sciutto, K. L., Hayley, S., Mount, M. P., O'Hare, M. J., Callaghan, S., Slack, R. S., Przedborski, S., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 13650–13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayley, S., Crocker, S. J., Smith, P. D., Shree, T., Jackson-Lewis, V., Przedborski, S., Mount, M., Slack, R., Anisman, H. & Park, D. S. (2004) J. Neurosci. 24, 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuura, K., Kabuto, H., Makino, H. & Ogawa, N. (1997) J. Neurosci. Methods 73, 45–48. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg, M. S., Fleming, S. M., Palacino, J. J., Cepeda, C., Lam, H. A., Bhatnagar, A., Meloni, E. G., Wu, N., Ackerson, L. C., Klapstein, G. J., et al. (2003) J. Biol. Chem. 278, 43628–43635. [DOI] [PubMed] [Google Scholar]
- 36.Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V. & Greenamyre, J. T. (2000) Nat. Neurosci. 3, 1301–1306. [DOI] [PubMed] [Google Scholar]
- 37.Sherer, T. B., Betarbet, R., Testa, C. M., Seo, B. B., Richardson, J. R., Kim, J. H., Miller, G. W., Yagi, T., Matsuno-Yagi, A. & Greenamyre, J. T. (2003) J. Neurosci. 23, 10756–10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Chiara, G. & Imperato, A. (1988) Proc. Natl. Acad. Sci. USA 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giros, B., Jaber, M., Jones, S. R., Wightman, R. M. & Caron, M. G. (1996) Nature 379, 606–612. [DOI] [PubMed] [Google Scholar]
- 40.Tatton, W. G., Kwan, M. M., Verrier, M. C., Seniuk, N. A. & Theriault, E. (1990) Brain Res. 527, 21–31. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi-Niki, K., Niki, T., Taira, T., Iguchi-Ariga, S. M. & Ariga, H. (2004) Biochem. Biophys. Res. Commun. 320, 389–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.