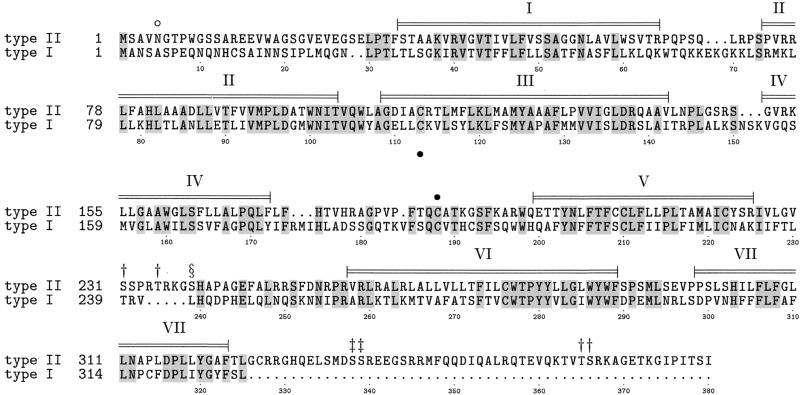
Figure 2.
Alignment of the amino acid sequences of the marmoset type II and human type I GnRH receptors. Conserved residues are shaded. α-helical regions predicted by homology modeling with the rhodopsin crystal structure are indicated. These helices will encompass the membrane spanning regions. A putative glycosylation site (○) and disulphide bridge (●) are indicated. Ser or Thr residues occurring in putative protein kinase C (†), casein kinase II (‡), and cAMP-/cGMP-dependent kinase (§) phosphorylation sites are indicated. Numerical residue annotation refers to marmoset type II sequence.