Fig. 3.
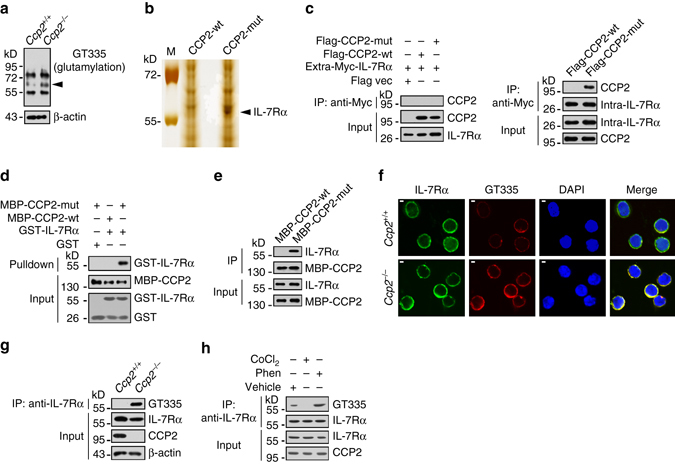
IL-7Rα is a substrate of CCP2 in CHILPs. a BM cells from WT and Ccp2 −/− mice were lysed. Protein glutamylation was examined with GT335 antibody by western blotting. Arrowhead denotes the differential band. b Recombinant CCP2-wt and enzymatic inactive CCP2 mutant (CCP2-mut) were immobilized with Affi-gel10 resin and assessed by addition of Ccp2 −/− BM lysates. The eluted fractions were resolved by SDS-PAGE, followed by silver staining. M: molecular weight marker. A differential band of ~60 kD appeared in CCP2-mut lane and was cut for mass spectrometry. The peptide sequences and coverage of IL-7Rα analyzed by LC-LTQ MS/MS are shown in the bottom graph. c Myc-tagged extracellular (amino acid: 21–239) or intracellular (amino acid: 265–459) segment of IL-7Rα and Flag-tagged CCP2-wt or CCP2-mut were co-transfected in 293 T cells for 36 h. Cell lysates were incubated with anti-Myc antibody for immunoprecipitation assay. IP immunoprecipitation. d GST-tagged intracellular segment of IL-7Rα (GST-IL-7Rα) was incubated with MBP-tagged CCP2-wt or CCP2-mut at 4 °C for 4 h, followed by incubation with GST beads. e CCP2-wt and CCP2-mut were incubated with BM lysates for pulldown assay. f CHILPs were incubated with GT335 and anti-IL-7Rα antibodies for immunofluorescence staining. IL-7Rα, green; GT335, red; nucleus, blue. Scale bar, 2 μm. g BM lysates from WT or Ccp2 −/− mice were immunoprecipitated with anti-IL-7Rα antibody, followed by immunoblotting. h WT BM cells were treated with CoCl2 or Phen. Cells were lysed and IL-7Rα glutamylation was assessed. Data represent four independent experiments
