Figure 5.
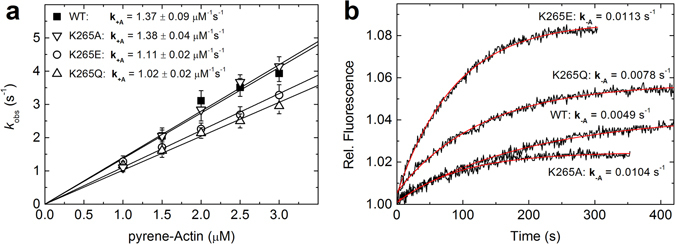
Binding and release of F-actin. Interaction of F-actin with WT and mutant myosin was followed using pyrene-labelled F-actin. (a) The actin concentration-dependent increase in the observed rate constants for pyrene-actin binding could be fit by a linear function. The slope defines the actin binding rate k +A, which is slightly decreased by mutations K265E and K265Q. Error bars give the standard deviation for three independent experiments. (b) Fluorescence transients observed after chasing pyrene-actin from the complex with myosin by excess unlabelled F-actin. Single exponential fits to the data define F-actin release rate constants, which are significantly increased for all three mutant myosins. All resulting kinetic parameters for F-actin binding and release are summarized in Table 2.
