Fig. 1.
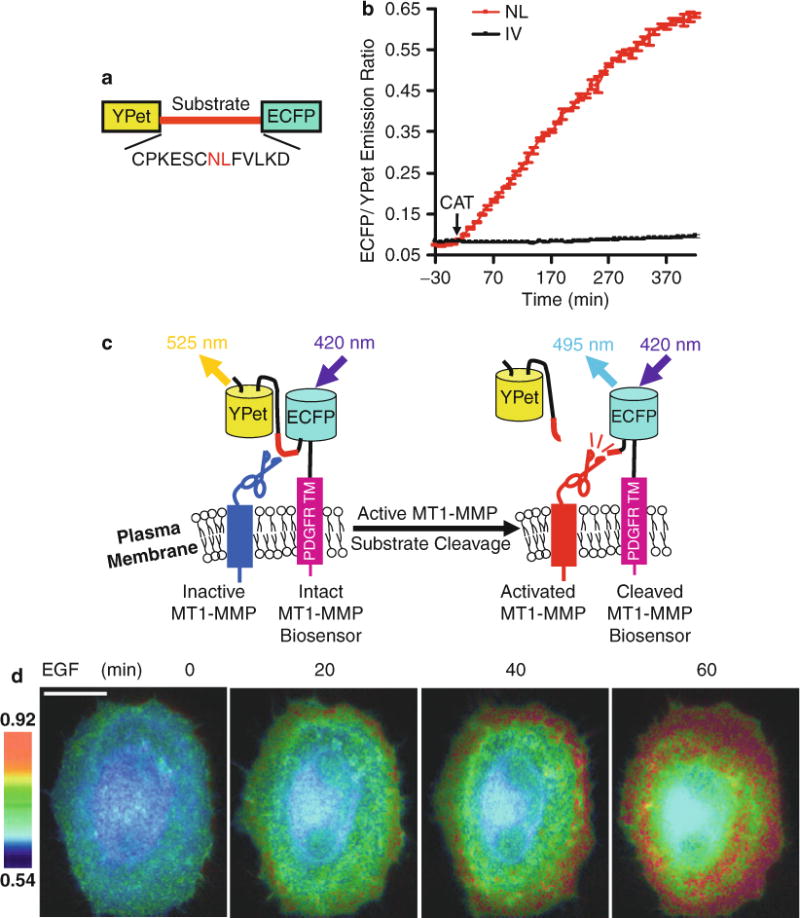
Design strategy of the MT1-MMP biosensor, and its FRET response in vitro and in mammalian cells. (a) Domain structure of MT1-MMP biosensor with YPet and ECFP at its N- and C-termini connected by a substrate peptide of MT1-MMP. (b) The time courses of ECFP/YPet emission ratio (mean ± SD) of wild-type (red line) and mutant (NL to IV mutation; black line) biosensors before and after incubation with the active catalytic domain of MT1-MMP (CAT). (c) The activation mechanism of the cell plasma membrane-tethered MT1-MMP biosensor. The biosensor is fused to the trans-membrane domain of PDGFR to position its sensing element outside of the plasma membrane, making it accessible to MT1-MMP. Active MT1-MMP can cleave the substrate peptide to separate ECFP and YPet, which leads to a decrease in FRET. (d) ECFP/YPet emission ratio images of a HeLa cell co-transfected with the MT1-MMP biosensor and MT1-MMP before and after EGF stimulation. Scale bar: 20 μm. This research was originally published in Journal of Biological Chemistry. Ouyang et al. Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging. J Biol Chem. 2008; 283(25):17740–8. © The American Society for Biochemistry and Molecular Biology
