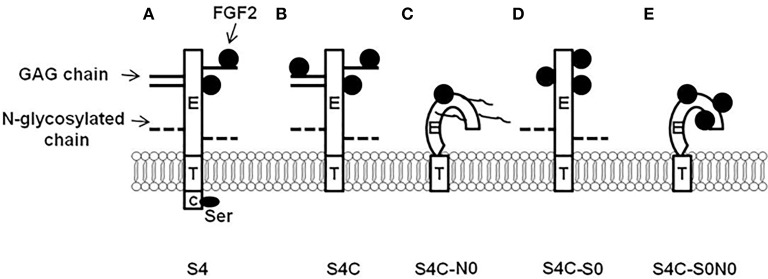
Figure 3.
Syndecan-4 cytoplasmic domain regulation of cellular responsiveness to fibroblast growth factor 2 (FGF2). (A) Syndecan-4 can bind FGF2 to its core protein and GAG chains, and then present FGF2 to its receptor to initiate cellular responsiveness to FGF2; (B) The deletion of syndecan-4 cytoplasmic domain may cause increased binding of FGF2 to syndecan-4 (Ser residue in the conserved 1 region is critical in this process). (C) The deletion of syndecan-4 N-linked glycosylated (N-glycosylation) chains changes the three dimensional structure of the core protein which may lead to the decreased interaction with FGF2; (D) The deletion of syndecan-4 glycosaminoglycan (GAG) chains gives more spaces for the core protein to bind to FGF2; and (E) The deletion of both syndecan-4 N-glycosylation chains and GAG chains changes the three dimensional structure of the core protein which deceases FGF2 binding to syndecan-4, however, the deletion of the GAG chains give more space for the core protein to bind to FGF2 which rescues the interaction between syndecan-4 and FGF2. S4, wild type syndecan-4; S4C, syndecan-4 without cytoplasmic domain; S4C-N0, syndecan-4 without cytoplasmic domain and N-glycosylation chains; S4C-S0, syndecan-4 without cytoplasmic domain and GAG chains; and S4C-S0N0, syndecan-4 without cytoplasmic domain, N-glycosylation chains, and GAG chains. (Figure reproduced from Song et al., 2012b).