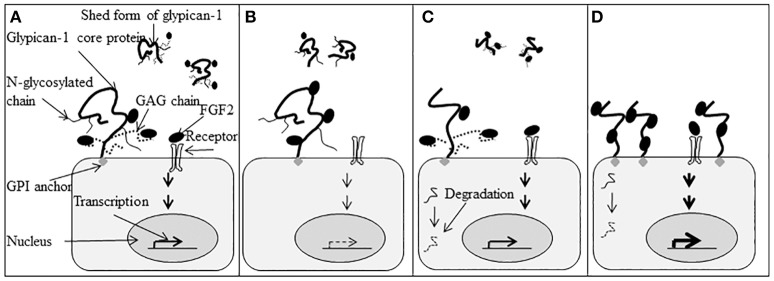
Figure 4.
Function of glypican-1 N-linked glycosylated (N-glycosylation) chains and glycosaminoglycan (GAG) chains in fibroblast growth factor 2 (FGF2) signaling. (A) Glypican-1 has both membrane-associated and shed forms. The membrane-associated form is attached to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor. Glypican-1 has three N-glycosylation chains and three GAG chains. The N-glycosylation chains are attached to the core protein at Asn76, Asn113, and Asn382. The Asn76 is referred to as the N1 chain, Asn113 is the N2 chain, and Asn382 is the N3 chain. Both glypican-1 core protein and GAG chains can bind and present FGF2 to its tyrosine kinase receptor and initiate cell signaling into the nuclei to stimulate gene transcription that influences cell proliferation and differentiation. (B) When GAG chains are deleted, glypican-1 core protein can still bind and present FGF2 to its tyrosine kinase receptor and initiate cell signaling. The cell has lower responsiveness to FGF2. (C) The deletion of glypican-1 N-glycosylation chains will change the three-dimensional structure of glypican-1 core protein which may influence glypican-1 localization on the cell membrane. Fibroblast growth factor 2 signaling will be initiated normally in the presence of the glypican-1 core protein and GAG chains. The protein without folding properly may be degraded ( ). (D) Glypican-1 N-glycosylation mutants without GAG chains cannot be released from the cell membrane normally. The increased number of glypican-1 core proteins bound to the cell surface will increase cell responsiveness to FGF2. Transcription levels are indicated by the density of the arrows (
). (D) Glypican-1 N-glycosylation mutants without GAG chains cannot be released from the cell membrane normally. The increased number of glypican-1 core proteins bound to the cell surface will increase cell responsiveness to FGF2. Transcription levels are indicated by the density of the arrows ( indicates normal transcription level,
indicates normal transcription level,  indicates reduced transcription level, and
indicates reduced transcription level, and  indicates increased transcription level). (Figure reproduced from Song et al., 2010).
indicates increased transcription level). (Figure reproduced from Song et al., 2010).