Figure 1.
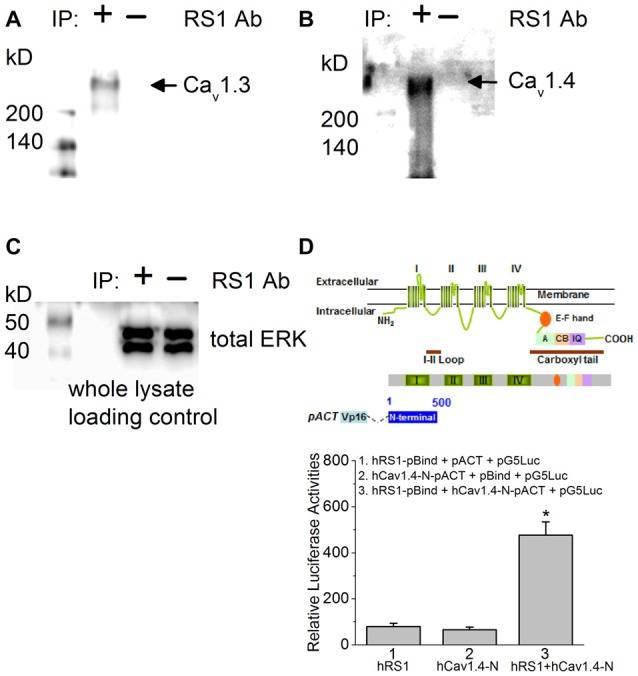
There is a physical interaction between retinoschisin (RS1) and L-type voltage-gated calcium channel (LTCC)α1 subunits. (A) Anti-RS1 antibody (RS1 Ab) is able to co-immunoprecipitate Cav1.3 from the porcine retina. (B) RS1 Ab is able to co-immunoprecipitate Cav1.4 from the porcine retina. (C) The whole cell lysates as loading control for (A,B). (D) Mammalian two-hybrid (luciferase reporter) assays show that hRS1 is able to interact with the first 500 amino acids from the N-terminus of Cav1.4 (hCav1.4-N) including the first motif (I). Cells co-transfected with hRS1 and hCav1.4-N (hRS1 + hCav1.4-N) have significantly higher luciferase activities than the other two control groups (n = 6 for each group, *p < 0.05, one-way ANOVA with Tukey’s post hoc tests. hRS1 vs. hRS1 + hCav1.4-N, p = 0.00000199; hCav1.4-N vs. hRS1 + hCav1.4-N, p = 0.00000126).
