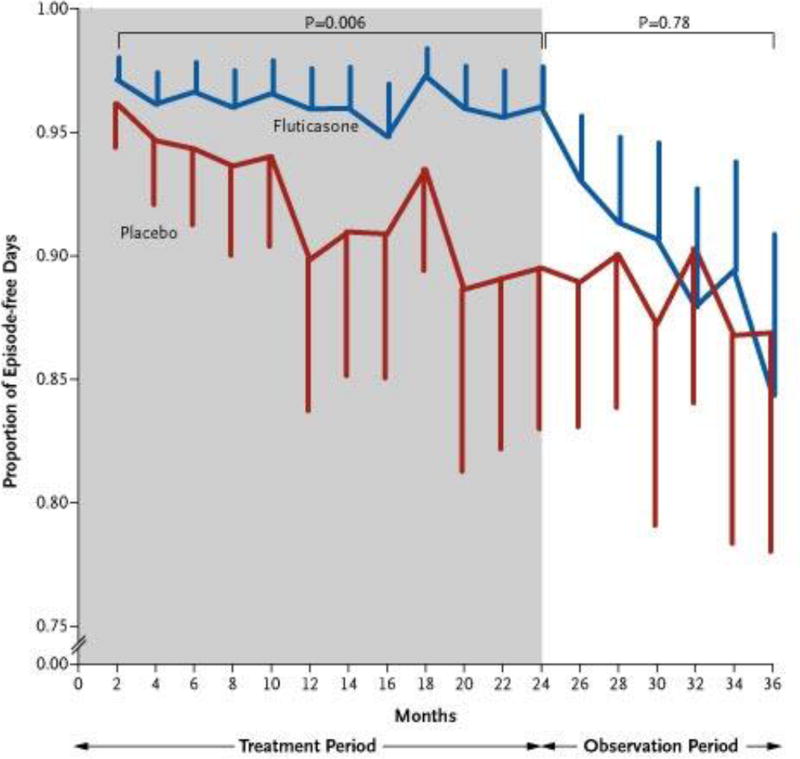
Figure 1. Proportion of Episode-free Days during the Treatment Period (indicated in gray) and the Observation Period (indicated in white).
While fluticasone did increase the proportion of episode-free days during the treatment period compared to placebo, this effect was lost during the observation period after treatment was discontinued. The listed p-values are for comparison between the treatment groups during the treatment and observation periods. The vertical bars represent 95% confidence intervals. (From New England Journal of Medicine, Guilbert, T. et al., Long-Term Inhaled Corticosteroids in Preschool Children at High Risk for Asthma, 354:1985-199. Copyright © (2006) Massachusetts Medical Society. Reprinted with permission. http://www.nejm.org/doi/full/10.1056/NEJMoa051378.)