Abstract
Virtual memory (VM) CD8+ T cells, which are present in unimmunized mice, yet possess T cell receptors specific for foreign antigens, have only been characterized in C57BL/6 mice. Here, we assessed the cytokine requirements for VM cells in C57BL/6 and BALB/c mice. As reported previously, VM cells in C57BL/6 mice rely mostly on IL-15 and marginally on IL-4. In stark contrast, VM cells in BALB/c mice rely substantially on IL-4 and marginally on IL-15. Further, NKT cells are the likely source of IL-4, because CD1d-deficient mice on a BALB/c background have significantly fewer VM cells. Notably, this NKT/IL-4 axis contributes to appropriate effector and memory T cell responses to infection in BALB/c mice, but not in C57BL/6 mice. However, the effects of IL-4 are manifest prior to, rather than during, infection. Thus, cytokine-mediated control of the precursor population affects the development of virus-specific CD8+ T cell memory.
Keywords: CD122, T cell memory, STAT6, IL-4
Introduction
Following their encounter with Ag in the context of appropriately stimulated Ag-presenting cells (APCs), naïve T cells undergo a process of clonal expansion and differentiation into effector cells. The effector T cell population is heterogeneous in that some effector T cells have “memory precursor” properties. Indeed, recent work has shown that, after acute infection, effector CD8+ T cells bearing increased CD127 (IL-7Rα) and decreased killer cell lectin-like receptor subfamily G, member 1 (KLRG1) have an increased propensity to generate memory T cells after adoptive transfer [1]. Conversely, KLRG1hiCD127low effector CD8+ T cells have less potential for memory T cell generation when transferred into recipient animals [1]. Thus, phenotypic heterogeneity defines effector cells that vary in their memory potential.
However, recent data showing heterogeneity in the naïve T cell compartment suggests an additional layer of depth to the above model by demonstrating that ∼5-50% of CD8+ T cells in unimmunized mice have a memory phenotype [2]. Although some of these T cells are likely specific for mouse flora or ingested food Ags, many of these cells develop independently from these factors [3] in the thymus, where they are enriched in mice that have specific TCR signaling defects, which include a SLP76 mutation and deficiencies in Itk, Id3, or CBP [4-8]. Hogquist and colleagues found that a population of IL-4-producing, PLZF+ lymphocytes was critical for the development of most of these memory phenotype (MP) cells [9]. Further, thymic MP cells were present in BALB/c, but not C57BL/6 mice, and were dependent upon CD1d, which is essential for thymic selection of PLZF-expressing NKT cells [9-11]. Thus, altered signaling within DP thymocytes enriches (either directly or indirectly) other innate immune PLZF-and IL-4-expressing lymphocytes that promote the thymic development of CD8+ T cells with a memory phenotype.
Notably, at least some MP cells are specific for particular foreign Ags, despite never being exposed to those Ags, leading to the term “virtual-memory” (VM) cells [3]. VM cells respond to Ag more quickly and have an enhanced ability to generate KLRG1lowCD127hi cells that enter the memory compartment at a higher rate than their naïve counterparts [3, 12]. In C57BL/6 mice, such VM cells can be identified by their low expression of CD49d, and in contrast to MP cells, are thought to develop in the periphery rather than the thymus. VM cells have been shown to depend largely on IL-15 transpresentation, and minimally on IL-4 [13, 14]. However, because VM cells have been studied exclusively within C57BL/6 mice, it remains unclear whether they exist in mice of other genetic backgrounds, particularly those that have high numbers of IL-4-producing, PLZF-expressing cells. In this report, we examine and compare the levels of VM cells and their dependence upon IL-4 and IL-15 in both BALB/c and C57BL/6 mice.
Results
More IL-4-dependent VM cells in BALB/c than in C57BL/6 mice
Virtual memory (VM) cells are cells within the MP pool that bear T cell receptors with specificity to foreign Ags not previously encountered by the host [3, 13, 14]. Although IL-4 contributes slightly to VM cells in C57BL/6 mice, IL-15 plays a dominant role in the generation/maintenance of these cells [13, 14]. However, the existence of VM cells, and their potential cytokine dependence in BALB/c mice, has not been established. To investigate this, we examined the levels and phenotype of Ag-specific VM CD8+ T cells in immunologically naive BALB/c mice lacking IL-4 and/or IL-15. To identify VM cells in BALB/c mice, we focused on cells specific for lymphocytic choriomeningitis virus (LCMV) (amino acids 118-126 from the nucleoprotein, restricted by Ld) using a “tetramer enrichment” technique [3, 13, 14].
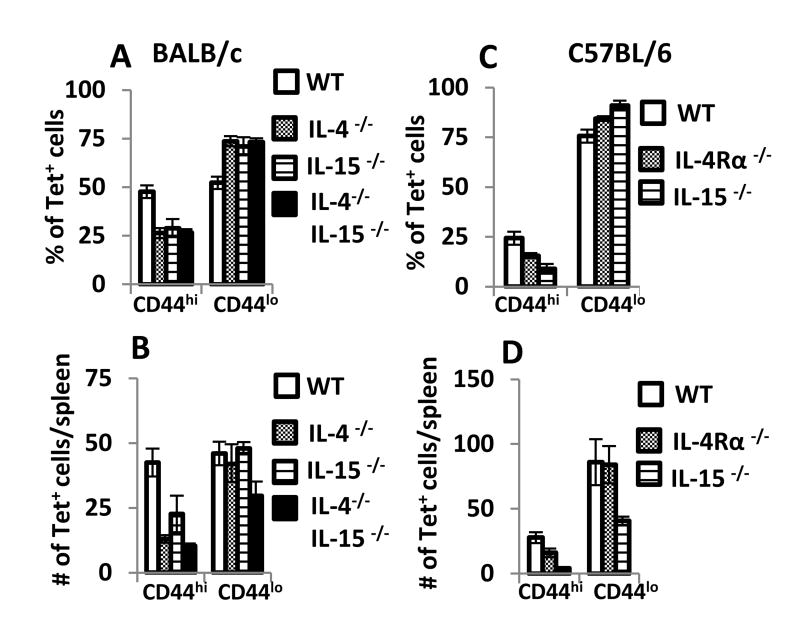
To identify VM cells in C57BL/6 mice, we focused on the LCMV epitope “gp33,” which contains the glycoprotein amino acids 33-41, restricted by Db. For both mouse strains, we also stained spleen cells with an anti-CD49d mAb, because low expression of CD49d marks VM cells [3, 13]. Compared to the flow-through cell fraction (tetramer-negative cells), we detected a significant number of VM cells in the bound fraction (enriched in tetramer-positive cells) from both C57BL/6 and BALB/c mice (supplementary figure 1); a significant percentage of these cells had increased expression of CD44 (Fig. 1A) and/or a second memory marker Ly6C (supplementary figure 1). The lack of either IL-4 or IL-15, or both, significantly reduced the frequency of LCMV-specific cells with a VM phenotype in BALB/c mice (Fig. 1A). Further, the overall number of CD44hi LCMV-specific VM cells was significantly decreased in the absence of IL-4 in mice on a BALB/c genetic background, while fewer of these cells were lost in these mice in the absence of IL-15 on the same background (Fig. 1B). Notably, neither IL-4 nor IL-15 appeared to reduce the frequency or number of viral-specific “naïve” cells (Fig.1A,B). The combined loss of IL-4 and IL-15 resulted in a slightly additional loss in total number of CD44hi viral-specific VM cells in BALB/c mice and a slight loss of viral-specific “naïve” CD44lo cells (Fig. 1B). In C57BL/6 mice, the loss of IL-4Rα signaling led to a slight, albeit significant decrease in the frequency and the total numbers of VM cells (Fig. 1C, D). In contrast, the loss of IL-15 led to a substantial reduction of the frequency and number of VM cells in C57BL/6 mice (Fig. 1C, D). However, the loss of IL-15 in a C57BL/6 genetic background also led to a slight loss of viral-specific “naïve” cells (Fig.1D). Thus, similar to the results for the MP compartment, the VM compartment in BALB/c mice relies more on IL-4 than IL-15, whereas the VM compartment in C57BL/6 mice relies heavily on IL-15 and minimally on IL-4.
Figure 1.
IL-4 and IL-15 both contribute to LCMV-sp. virtual memory (VM) CD8+ T cells in BALB/c mice. Groups of BALB/c and C57BL/6 mice (N=5-6/group) were sacrificed and CD8+ T cells were negatively enriched from single spleen cell suspensions with a magnetic column (Miltenyi Biotech), stained with PE-labeled MHC-tetramers and then enriched over an anti-PE column, after which cells were analyzed by flow cytometry. Results show the (A, C) frequency of tetramer+ cells that are CD44hi and (B, D) the total number of tetramer+ (Ldnp118-sp.) CD8+ T cells from BALB/c wild-type, IL-4-/-, IL-15-/-, and IL-4-/- IL-15-/- mice as well as (Dbgp33-sp.) CD8+ T cells from C57BL/6 wild-type, IL-15-/- and IL-4Rα-/- mice. Data are pooled from 4-5 independent experiments.
IL-4 contributes to effector and memory CD8+ T cell responses in BALB/c, but not C57BL/6, mice
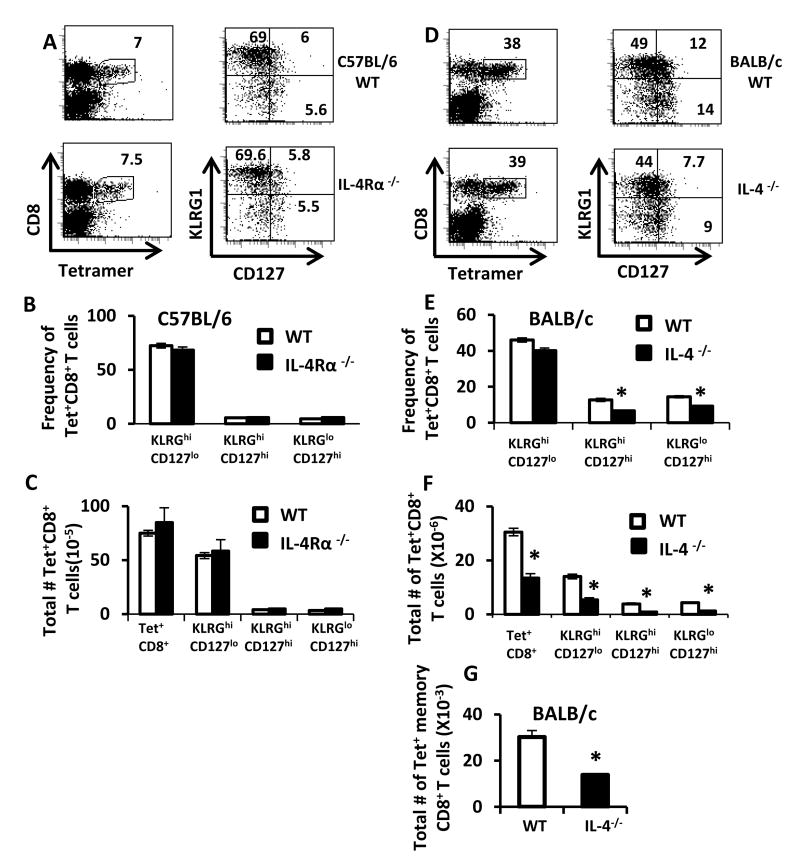
Because IL-4 differently controlled VM cells in C57BL/6 vs. BALB/c mice, we evaluated whether this differential effect would alter subsequent immune responses. To do this, we first infected groups of C57BL/6 background wild-type and IL-4Rα-/- mice with LCMV, sacrificed them 8 days later and examined the expression of KLRG-1 and CD127 on LCMV-specific CD8+ T cells that were identified by their binding to Db-gp33 tetramers. The frequency and numbers of overall Dbgp33-sp. effector T cells as well as effector (KLRG1hiCD127low) and pre-memory (KLRG1lowCD127hi) subsets were indistinguishable between C57BL/6 IL-4Rα-/- and wildtype C57BL/6 controls (Fig. 2A-C). The lack of an effect of IL-4Rα-signaling on antiviral CD8+ T cell responses was also observed on day 24 after infection (supplementary figure 2). Further, similar negative data were obtained in IL-4-/- mice on a C57BL/6 background (data not shown). These data show that, in C57BL/6 mice, endogenous IL-4 is not critical for development of normal effector T cell responses after LCMV infection.
Figure 2.
IL-4 contributes to effector and memory CD8+ T cell responses to LCMV in BALB/c, but not C57BL/6 mice. (A). Groups of C57BL/6 wild-type and IL-4Rα-/- mice (N=5/group) were infected i.p. with LCMV and were sacrificed on day 8 after infection. Spleen cells were stained with Dbgp33-tetramer along with antibodies against KLRG1 and CD127. Results show Dbgp33-sp. T cells (left panels) and expression of KLRG1 and CD127 (right panels) on cells from C57BL/6 wild-type (top panels) and IL-4Rα-/- mice (bottom panels). (B, C) Graphs show the frequency and total numbers of effector CD8+ T cell subsets. (D-G) Groups of BALB/c wild-type and IL-4-/- mice were infected i.p. (N = 5) with LCMV and sacrificed on (D-F) day 8 or (G) day 60 after infection. Spleen cells were stained with Ldnp118 tetramers and antibodies against CD8, KLRG1 and CD127. Results show Ldnp118-sp. T cells (D, left panels) and expression of KLRG1 and CD127 (D, right panels) on BALB/c wild-type (D, top panels) and IL-4-/- mice (D, bottom panels). (E, F) Graphs show the frequency and total numbers of effector CD8+ T cell subsets. (G) Results show the total numbers of Ld-np118-sp. CD8+ central memory (CD44hiCD62Lhi) T cells on day 60 after infection. Data are representative of 3 independent experiments.
We next examined the role of IL-4 in promoting effector and memory CD8+ T cell responses to LCMV in BALB/c mice. Cohorts of BALB/c IL-4-sufficient or deficient mice were infected with LCMV and their LCMV-specific CD8+ T cell responses were tracked using Ldnp118-126 tetramers, which identify the dominant LCMV epitope in BALB/c mice. At the peak of the response, roughly 40% of all of the CD8+ T cells stain with the Ldnp118 tetramer; this is IL-4-independent (Fig. 2D). We also examined effector CD8+ T cell sub-populations in LCMV-infected BALB/c IL-4-sufficient and deficient mice. Eight days after infection, IL-4-deficient mice had significantly reduced frequencies of both KLRG1lowCD127hi and KLRG1hiCD127hi cells (Fig. 2D-F). Further, the overall numbers of all subsets of LCMV-sp. CD8+ T cells were significantly reduced in IL-4-/- mice (Fig. 2F). There was no difference in viral load between WT BALB/c and IL-4-/- mice on a BALB/c background (data not shown). In addition, similarly decreased cell frequencies and numbers in IL-4-/- BALB/c mice were maintained through day 24 after infection (supplementary figure 2), showing that this effect was not likely due to a difference in kinetics of the response. Further, this deficit in LCMV-sp. CD8+ T cells persisted into the memory stage, as the overall numbers of Ld-np118-sp. central memory CD8+ T cells (CD44hiCD62Lhi) were significantly reduced 60 days after infection (Fig. 2G). Thus, IL-4-deficient mice on a BALB/c background have reduced effector and memory CD8+ T cell responses to LCMV.
IL-4 manifests its effects on memory cell development prior to infection
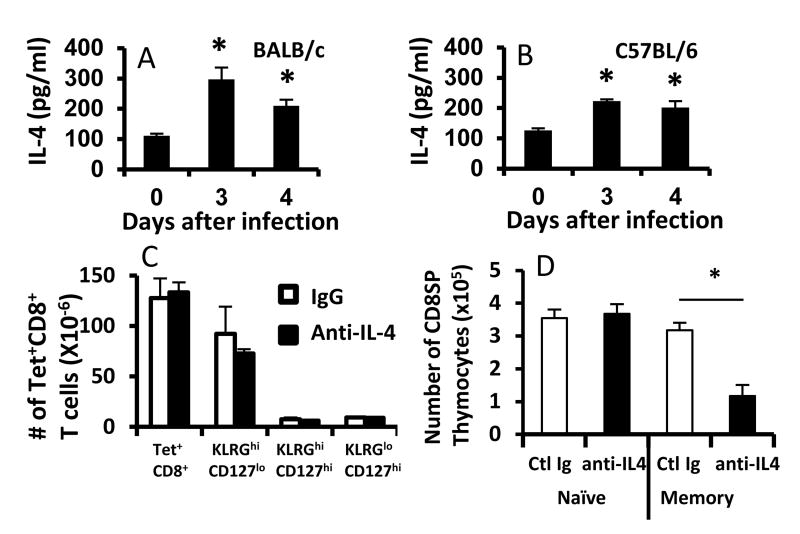
Because LCMV drives an IFN-γ-dominated response (even in BALB/c mice), the effects of IL-4 in the model were surprising. To determine whether IL-4 was induced during LCMV infection and could therefore influence the developing T cell response, we assessed serum IL-4 levels using a sensitive cytokine capture assay. IL-4 secretion was slightly, but similarly increased in C57BL/6 and BALB/c mice 3 and 4 days after LCMV infection (Fig. 3A, B). By day 8, IL-4 levels were no different between LCMV-infected and control uninfected C57BL/6 and BALB/c mice (data not shown). We also examined the levels of CD124 (IL-4Rα) on antigen-specific CD8+ T cells at the peak of the response. While CD124 levels were increased after activation, there was only a slight (∼1.5fold) difference in the levels of CD124 between KLRG1loCD127hi cells and other populations of effector cells (Supplementary figure 3). Nonetheless, to determine whether this transient, low-level increase in IL-4 contributed to the altered effector phenotype observed after infection, we neutralized IL-4 in vivo during the first week of LCMV infection in BALB/c mice and assessed its effect on the generation of effector CD8+ T cell responses. Neither the frequencies (not shown), nor the numbers, of LCMV-sp. CD8+ T cells (and relevant subsets) were altered by IL-4 neutralization (Fig. 3C), whereas anti-IL-4 treatment significantly decreased bulk memory phenotype CD8+ T cells in the thymus (Fig. 3D), confirming the functionality of the anti-IL-4 antibody. Thus, the effect of endogenous IL-4 on the CD8+ T cell response to LCMV was not due to IL-4 produced during infection.
Figure 3.
IL-4 promotion of effector and memory CD8+ T cell responses occurs prior to infection. (A, B) Groups of BALB/c and C57BL/6 mice (N = 5/group) were either uninfected or were infected with LCMV and injected i.v. with 10 μg of biotinylated-anti-IL-4 mAb on either day 3 or day 4 and then bled one day later (day 0 values refer to uninfected mice). IL-4 secretion was determined by IVCCA ELISA as described [30]. (C) Groups of BALB/c mice (N=6/group) were infected with LCMV and were injected i.p. with 1 mg of either isotype control (clone J1.2) or anti-IL-4 (clone BVD4-1D11.2) antibody on days 0, 2, 4, 6 and sacrificed on day 8 after infection. Splenocytes were stained with Ldnp118 tetramers and antibodies against CD44, KLRG1, and CD127. Results show the total numbers of Ldnp118-sp. T cells as well as effector and pre-memory subsets. (D) Groups of BALB/c mice (N=4/group) received 1 mg of either isotype control antibody or anti-IL-4 antibody on days 0, 3, 6, 9, 12 and were sacrificed on day 14. Thymocytes were stained with antibodies against CD4, CD8, CD44, and Ly6C. Naïve cells were identified as CD8+CD4-CD44loLy6Clo and memory cells were identified as CD8+CD4-CD44hiLy6Chi. Results show that anti-IL-4 significantly reduced the total number of CD8 SP memory phenotype thymocytes. * denotes p<0.003, student's t-test.
CD1d promotes virtual memory cells and antigen-specific CD8+ T cell responses in BALB/c mice
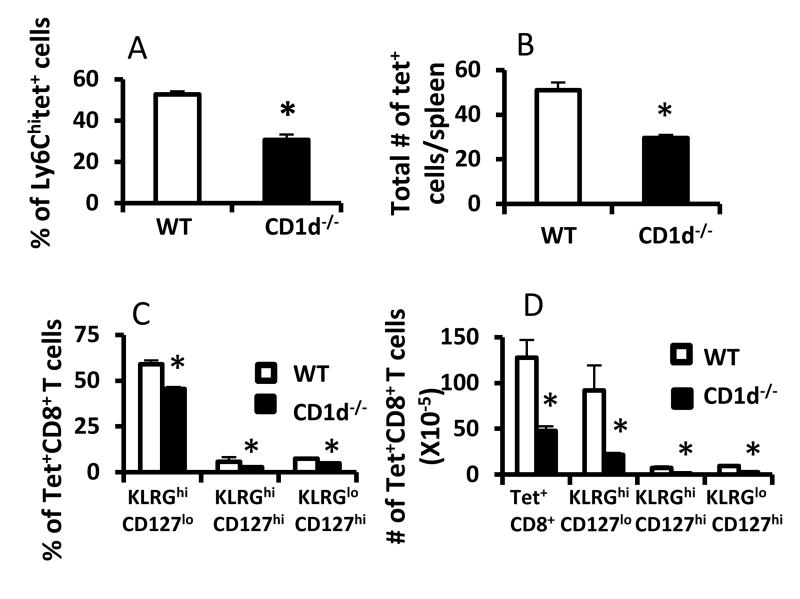
We next used CD1d-/- mice on a BALB/c background to independently determine whether the effects of IL-4 on the immune response to LCMV are exerted during or prior to infection. CD1d-/- mice lack IL-4-producing NKT cells in the thymus and consequently, have a significant reduction in MP cells [9]. Given the requirement for IL-4 in promoting VM cells in BALB/c mice (Fig. 1), we reasoned that, similar to MP cells, VM cells in BALB/c mice should be dependent upon CD1d-restricted cells. As expected, CD1d-/- mice had significantly fewer PLZF+ cells in the thymus than wildtype BALB/c mice (supplementary figure 4). Consistent with this, numbers of VM cells in CD1d-/- BALB/c mice were significantly reduced relative to WT controls (Fig. 4A, B). For VM cells, we found better separation of the memory population using Ly6C as a marker, relative to CD44. Following LCMV infection, CD1d-/- mice on a BALB/c background also had significant reduction in frequencies and total numbers of tetramer+ cells including effector and pre-memory cells (Fig. 4C, D). This effect was independent of IL-4 produced during infection, because IL-4 levels in both BALB/c wild-type and CD1d-/- mice increased 1.5 fold on day 4 after infection (not shown). Thus, similar to the effects of IL-4, CD1d likely contributes to the development of effector and memory BALB/c CD8+ T cell responses through its effects on the precursor compartment.
Figure 4.
CD1d promotes LCMV-specific precursors and pre-memory cells. (A, B) Groups of BALB/c wild-type and CD1d-/- mice were sacrificed and the numbers of LCMV-sp. T cells were enumerated via tetramer enrichment. Results show a significant loss of percent and total number of Ldnp118-sp. precursor cells in CD1d-/- relative to wild-type BALB/c mice (p<0.05). (C, D). Groups of BALB/c wild-type and CD1d-/- mice were infected with LCMV and sacrificed 8 days later. Splenocytes were stained with Ldnp118 tetramers and antibodies against CD44, KLRG1, and CD127. Results show the total numbers of Ldnp118-sp. T cells as well as percent and total numbers of effector and pre-memory subsets and are representative of 2 independent experiments.
Discussion
Here we report, for the first time, the characterization of VM cells (MP cells with specificity for foreign Ag) in BALB/c mice. Our data show that VM cells in BALB/c mice are largely IL-4 dependent and minimally IL-15 dependent. This is in contrast to prior studies that have shown a relatively modest role for IL-4, but a dominant role of IL-15 in VM development in C57BL/6 mice [13-15], similar to our results here in C57BL/6 mice. In these prior studies, it was suggested that the VM cells acquired their phenotype during the peripheral homeostatic proliferative response to the lymphopenia that exists in neonatal mice. In contrast, we found a dominant role for IL-4 and a partial role for IL-15 in promoting VM cells in BALB/c mice. The strong IL-4 dependence of BALB/c, but not C57BL/6, MP cells and VM cells suggests that VM cells, like MP cells, develop in the thymus of BALB/c mice. We have not been successful, however, at assessing VM cells in BALB/c thymus. We think that differences in the sheer numbers of available T cells in the thymus vs the periphery is probably the reason for this, because the peripheral compartment is most likely the cumulative sum of thymic output over time. Consequently, the spleen should have many more antigen-specific precursors than exist at any given timepoint in the thymus. Nonetheless, our data suggest that, in BALB/c mice, VM cells acquire their memory phenotype in the thymus.
One question that arises from this work is whether the effects of IL-4 require IL4Rα expression on CD8+ T cells themselves. Analysis of bulk memory phenotype cells from CD4Cre-IL-4Rαf/- mice showed that T cell expression of IL-4Rα is critical for the homeostasis of overall memory phenotype cells (data not shown). In addition, another group has shown, using a mixed bone marrow chimera approach, that T cells lacking IL-4Rα have a substantial loss of bulk memory phenotype cells [16]. Further, the Jameson group has found, using a mixed bone marrow chimera approach, that IL-4Rα expression on CD8+ T cells is critical for their development into virtual memory cells (personal communication).
A second question that arises around this work is why the reduction in VM cells in the absence of IL-4 and/or IL-15 is not complete. This suggested that IL-4 and IL-15 are not the only regulators of VM cell homeostasis. We reasoned that IL-7, a cytokine well known to control the survival of peripheral T cells, may contribute to VM cell homeostasis and partially compensate for the loss of IL-4 and IL-15. Consistent with this possibility, IL-7 neutralization for two weeks led to a slight loss of VM cells in WT cells and a more dramatic loss of VM cells in IL-4/-15-double deficient mice (Supplementary figure 5). Notably, in both WT and IL-4/-15-double deficient mice, viral-specific “naïve” cells were reduced with anti-IL-7 neutralization (Supplemental figure 5). Together, these data show that IL-7 also contributes to VM cell homeostasis in addition to IL-4 and IL-15.
The existence of VM cells within the “naïve” repertoire is intriguing in light of current models of antigen-specific memory T cell development. Adoptively transferred VM cells proliferate more rapidly after antigen challenge in vivo relative to transferred naïve T cells [3, 12, 13]. Our data are consistent with these prior studies and show that the reduced number of precursor cells in IL-4-/- BALB/c mice negatively impacts subsequent effector and memory responses. Indeed, dose response studies using TCR Tg mice suggest that up to a certain threshold, the more TCR Tg T cells transferred, the larger the magnitude of the response [17]. Further, using a TCR Tg system, it was also shown that VM cells more rapidly re-enter the memory pool than their naïve counterparts [12]. The fact that both memory precursors and central memory cells were reduced in IL-4-/- BALB/c mice after LCMV infection and that IL-4-neutralization did not recapitulate this result, shows that the effects of IL-4 were manifest prior to infection, a result of the decreased numbers of precursor cells. Colleagues of ours have reached a similar conclusion independent of this work (S. Jameson, personal communication). Thus, the size of the VM compartment can be influenced prior to infection, by the genetic background of the host.
One important question in this regard is whether VM cells exist in humans. Consistent with this possibility, a few recent reports demonstrate tetramer-based enrichment of antigen-specific CD8+ T cells from individuals who were unlikely to have ever been exposed to those antigens [18-21]. However, the frequency of memory phenotype cells has varied widely in these studies [18-21]. Further work will be necessary to definitively address this question and to determine whether putative human VM cells are consistently IL-4- and/or IL-15-dependent or vary in this regard in different individuals. There are data in humans demonstrating the existence of MP T cells within the CD8SP thymocyte pool. Although the authors of those studies suggest that CD8SP T cell recognition of MHC II on thymocytes is important for the acquisition of a MP [22], the IL-4-dependence of this phenotype in humans has not been elucidated. Thus, it remains possible that the development of thymic MP cells in BALB/c mice may be representative of similar cells in humans. We are currently trying to determine the gene expression patterns of IL-4-dependent versus IL-15-dependent VM cells with an eye to defining a signature of each type of population. Further understanding of such differences could be exploited to impact the development of protective effector/memory responses, and, as such, could have implications for vaccine responsiveness.
Materials and methods
Mice and viral infection
C57BL/6 and BALB/c mice were either purchased from Taconic Farms or were bred in house. IL-4-/- and IL-4Rα-/- mice on a C57BL/6 background were as described [23]. IL-4-/- mice on a BALB/c background (received from Dr. Renate Morwitz, NIH) have been described [24]. IL-15-deficient mice were originally purchased from Taconic labs and were backcrossed to BALB/c mice for at least ten generations prior to use in experiments. IL-4-/-IL-15-/- mice on a BALB/c background were generated by breeding IL-4-/- to IL-15-/- mice and then intercrossing the F1 generation and screening for IL-4-/-IL-15-/- mice by PCR. CD1d-/- mice on a BALB/c background were purchased from Jackson Labs. dLckCre mice (a generous gift of Dr. Nigel Killeen, University of California San Francisco) were bred to Stat5fl/fl mice (a generous gift of Dr. Lothar Heninghausen, NIH), both of which we have described previously [25-27]. All mice were used between 3-8 months of age.
Mice were infected intraperioneally (i.p.) with 2×105 pfu of the Armstrong strain of lymphocytic choriomeningitis virus (LCMV). LCMV was grown in BHK-21 cells and viral titers from spleen and liver homogenates were determined by plaque assay on Vero cell monolayers as described [28]. Animals were housed under specific pathogen-free conditions in the Division of Veterinary Services and experimental procedures were reviewed and approved by the institutional animal care and use committee (IACUC) at the Cincinnati Children's Hospital Research Foundation.
MHC Tetramer staining and flow cytometry
Single spleen cell or thymocyte suspensions (∼2 million cells) were stained with different combinations of the following Abs to cell surface Ags: anti-CD4, CD8, CD44, KLRG1, CD127, Ly6C, CD122, CD124, TCRβ. Cells were also stained intracellularly with Abs against PLZF (from either BD Biosciences or eBioscience) along with surface staining with class I MHC tetramers (Ldnp118-126, Dbgp33-4, and CD1d-PBS57). The Ldnp118 tetramer and the CD1d-PBS57 tetramer were produced by the NIH Tetramer Core and the Dbgp33 tetramers were generated in E. coli and were coupled to either APC or to PE [29]. Data were collected on an LSRII flow cytometer and analyzed using FacsDIVA software.
MHC Tetramer enrichment
Ag-specific T cells were enriched by a recently described method [3]. Briefly, 3×108 splenocytes were suspended in HBSS buffer (2% FCS and 0.5% 24G2 supernatant). Cells were stained with tetramer for 60 min at 4°C, then washed, resuspended in sorting buffer (HBSS with 0.5% BSA and 2mM EDTA), and stained with anti-PE – coupled MACS MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 30 min with occasional shaking at 4°C. Cells were then washed, resuspended in sorting buffer, and the tetramer bound cells enriched on a magnetized MACS column (Miltenyi Biotec). After elution, cells were centrifuged and resuspended in FACS buffer and stained for markers used to enhance gating of tetramer+ CD8+ T cells (CD8 and CD3 as a positive gate; B220, CD4, CD11b and CD11c as a dump gate), as well as with Abs to determine activation status (CD44, CD49d, CD122, Ly6C). Flow cytometry of stained cells was performed on an LSRII (BD Biosciences) and data were analyzed using FacsDIVA (BD Biosciences) and in some cases FlowJo (Ashland, OR) software.
In vivo IL-4 measurement and neutralization
Groups of BALB/c and C57BL/6 mice were either uninfected or were infected with LCMV and injected i.v. with 10 μg of biotinylated-anti-IL-4 mAb on day 3 or 4 and then bled on day 4 or 5, respectively, after infection. Levels of serum IL-4 were determined by IVCCA ELISA [30]. For IL-4 neutralization, groups of mice were injected i.p. with either 1 mg of anti-IL-4 or isotype control mAb on days 0, 2, 4, and 6 relative to infection with LCMV and sacrificed on day 8 after infection.
Statistical Analyses
Statistical analyses were performed using Student's t-test with Microsoft Excel or with Minitab for Windows Software (Release 14), State College, Pennsylvania.
Supplementary Material
Acknowledgments
The authors thank Dr. Sing Sing Way for help developing the “tetramer enrichment” protocol, the NIH tetramer core for MHC tetramers, and Drs. Harinder Singh, Lee Grimes, and Chris Karp, and members of the Finkelman, Hildeman, and Morris Labs for helpful discussion. This work was supported by Public Health Service Grants RO1AI057753 (D.A.H.) and R01AI070300 (F.D.F.) a Department of Veterans Affairs Merit Award (F.D.F); and in part by PHS Grant P30 DK078392 (Research Flow Cytometry Core) of the Digestive Disease Research Core Center and P30 DK090971 of the Hematology Center of Excellence in Cincinnati.
Footnotes
Conflict of Interest disclosure: The authors declare no commercial or financial conflict of interest.
References
- 1.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Fukuyama T, Kasper LH, Boussouar F, Jeevan T, van Deursen J, Brindle PK. Histone acetyltransferase CBP is vital to demarcate conventional and innate CD8+ T-cell development. Mol Cell Biol. 2009;29:3894–3904. doi: 10.1128/MCB.01598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci U S A. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosinowski T, White JT, Cross EW, Haluszczak C, Marrack P, Gapin L, Kedl RM. CD8alpha+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J Immunol. 2013;190:1936–1947. doi: 10.4049/jimmunol.1203149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurzweil V, LaRoche A, Oliver PM. Increased peripheral IL-4 leads to an expanded virtual memory CD8+ population. J Immunol. 2014;192:5643–5651. doi: 10.4049/jimmunol.1301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Chen Q, Nguyen T, Yu Q, Sen JM. T cell factor-1 and beta-catenin control the development of memory-like CD8 thymocytes. J Immunol. 2012;188:3859–3868. doi: 10.4049/jimmunol.1103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alanio C, Lemaitre F, Law HK, Hasan M, Albert ML. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 2010;115:3718–3725. doi: 10.1182/blood-2009-10-251124. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt J, Neumann-Haefelin C, Altay T, Gostick E, Price DA, Lohmann V, Blum HE, Thimme R. Immunodominance of HLA-A2-restricted hepatitis C virus-specific CD8+ T cell responses is linked to naive-precursor frequency. J Virol. 2011;85:5232–5236. doi: 10.1128/JVI.00093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su LF, Davis MM. Antiviral memory phenotype T cells in unexposed adults. Immunol Rev. 2013;255:95–109. doi: 10.1111/imr.12095. [DOI] [PubMed] [Google Scholar]
- 21.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris SC, Heidorn SM, Herbert DR, Perkins C, Hildeman DA, Khodoun MV, Finkelman FD. Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J Immunol. 2009;182:1429–1438. doi: 10.4049/jimmunol.182.3.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 25.Raynor J, Sholl A, Plas DR, Bouillet P, Chougnet CA, Hildeman DA. IL-15 Fosters Age-Driven Regulatory T Cell Accrual in the Face of Declining IL-2 Levels. Front Immunol. 2013;4:161. doi: 10.3389/fimmu.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripathi P, Kurtulus S, Wojciechowski S, Sholl A, Hoebe K, Morris SC, Finkelman FD, Grimes HL, Hildeman DA. STAT5 is critical to maintain effector CD8+ T cell responses. J Immunol. 2010;185:2116–2124. doi: 10.4049/jimmunol.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang DJ, Wang Q, Wei J, Baimukanova G, Buchholz F, Stewart AF, Mao X, Killeen N. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J Immunol. 2005;174:6725–6731. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
- 28.Hildeman D, Yanez D, Pederson K, Havighurst T, Muller D. Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. J Virol. 1997;71:9672–9678. doi: 10.1128/jvi.71.12.9672-9678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris SC, Coffman RL, Finkelman FD. In vivo IL-4 responses to anti-IgD antibody are MHC class II dependent and beta 2-microglobulin independent and develop normally in the absence of IL-4 priming of T cells. J Immunol. 1998;160:3299–3304. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.