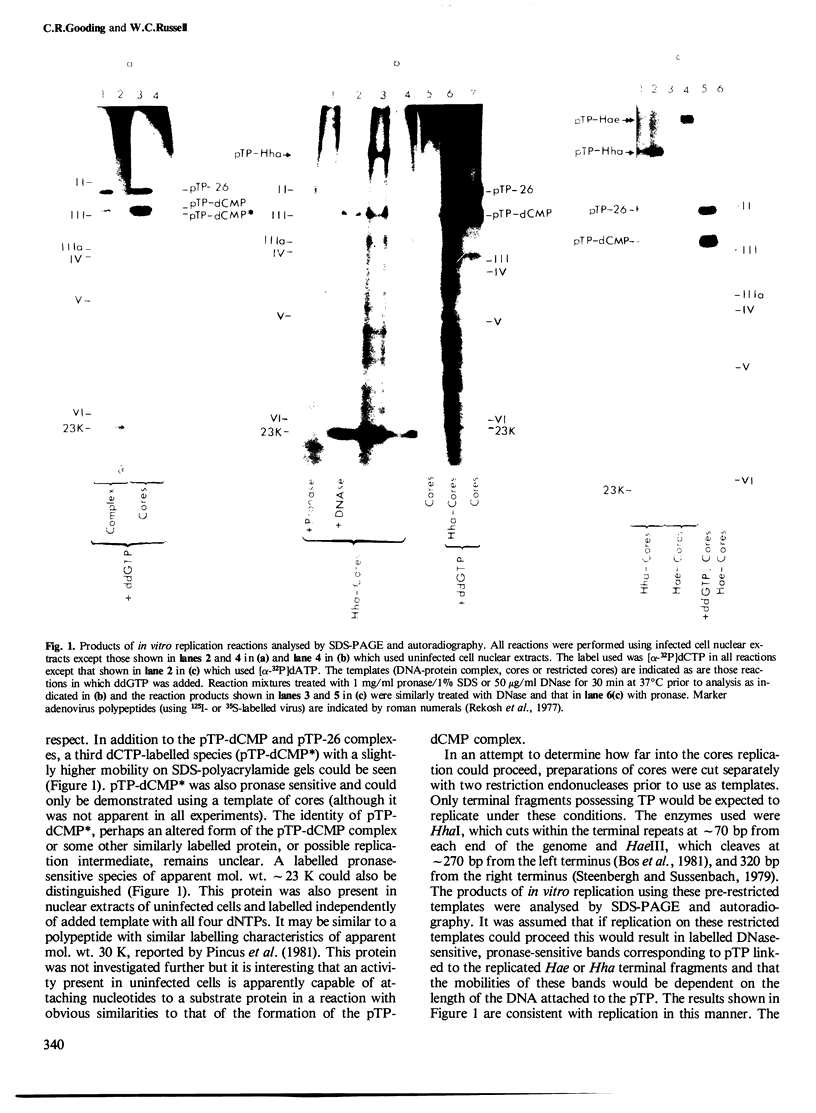
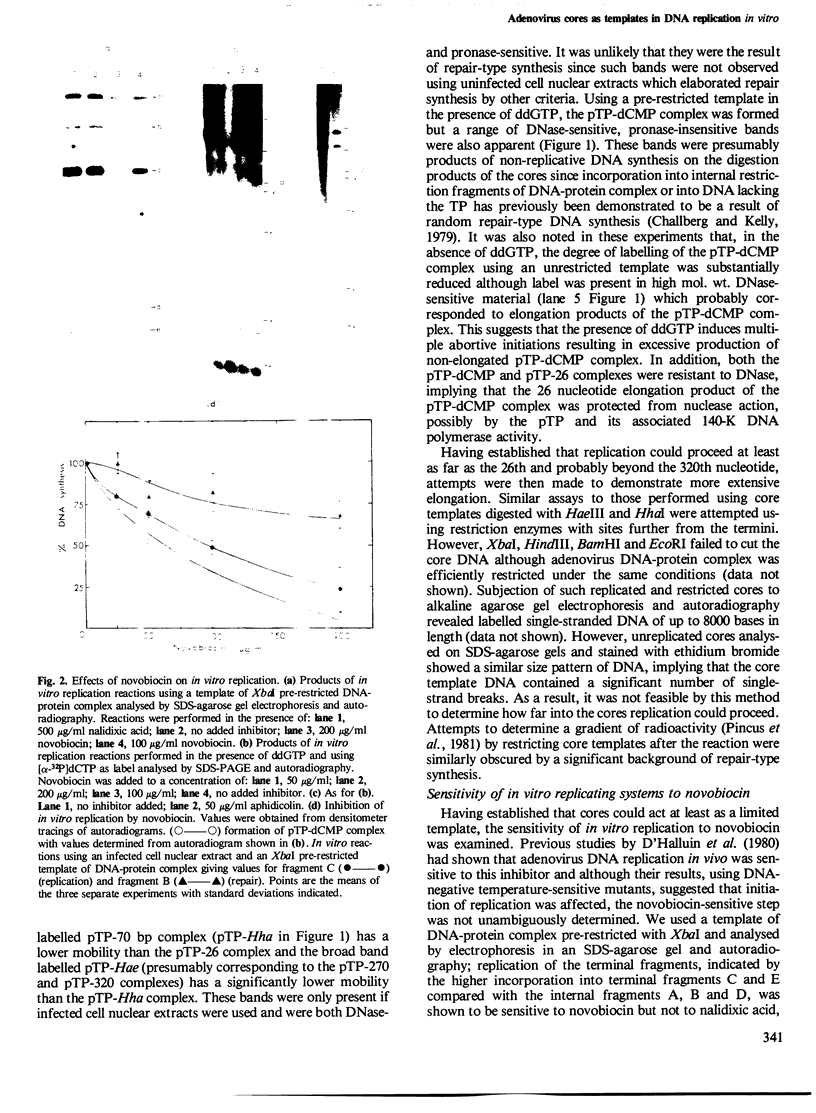
Abstract
Adenovirus cores prepared by gentle disruption of virus by heating at 56 degrees C in the presence of deoxycholate were able to function as templates in an in vitro DNA replication system, allowing both initiation, indicated by the formation of terminal protein-dCMP complex, and elongation of > 300 nucleotides. Using both cores and DNA-protein complexes as templates, it was also demonstrated that novobiocin, an inhibitor of DNA gyrase, inhibited in vitro DNA replication by preventing formation of the initiation complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldi M. I., Benedetti P., Mattoccia E., Tocchini-Valentini G. P. In vitro catenation and decatenation of DNA and a novel eucaryotic ATP-dependent topoisomerase. Cell. 1980 Jun;20(2):461–467. doi: 10.1016/0092-8674(80)90632-7. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Loucheux-Lefevbre M. H. Structure of adenovirus nucleoprotein core studied by circular dichroism and selective radiochemical labeling. Biochem Biophys Res Commun. 1982 Jul 30;107(2):470–480. doi: 10.1016/0006-291x(82)91515-7. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Ostrove J. M., Kelly T. J., Jr Initiation of adenovirus DNA replication: detection of covalent complexes between nucleotide and the 80-kilodalton terminal protein. J Virol. 1982 Jan;41(1):265–270. doi: 10.1128/jvi.41.1.265-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. Effects of novobiocin on adenovirus DNA synthesis and encapsidation. Nucleic Acids Res. 1980 Apr 11;8(7):1625–1641. doi: 10.1093/nar/8.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Novobiocin inhibition of simian virus 40 DNA replication. Nature. 1980 Jul 31;286(5772):529–531. doi: 10.1038/286529a0. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Sugino A. Novobiocin and nalidixic acid target proteins in yeast. Biochem Biophys Res Commun. 1980 Sep 16;96(1):306–312. doi: 10.1016/0006-291x(80)91215-2. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Harpst J. A., Russell W. C. Electron microscopy of adenovirus cores. J Gen Virol. 1975 Jul;28(1):49–58. doi: 10.1099/0022-1317-28-1-49. [DOI] [PubMed] [Google Scholar]
- Pincus S., Robertson W., Rekosh D. Characterization of the effect of aphidicolin on adenovirus DNA replication: evidence in support of a protein primer model of initiation. Nucleic Acids Res. 1981 Oct 10;9(19):4919–4938. doi: 10.1093/nar/9.19.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier G. G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Ruben M., Bacchetti S., Graham F. Covalently closed circles of adenovirus 5 DNA. Nature. 1983 Jan 13;301(5896):172–174. doi: 10.1038/301172a0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Hayashi K., Sanderson P. J., Pereira H. G. Adenovirus antigens--a study of their properties and sequential development in infection. J Gen Virol. 1967 Oct;1(4):495–507. doi: 10.1099/0022-1317-1-4-495. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Patel G., Precious B., Sharp I., Gardner P. S. Monoclonal antibodies against adenovirus type 5: preparation and preliminary characterization. J Gen Virol. 1981 Oct;56(Pt 2):393–408. doi: 10.1099/0022-1317-56-2-393. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Sussenbach J. S. The nucleotide sequence of the right-hand terminus of adenovirus type 5 DNA: implications for the mechanism of DNA replication. Gene. 1979 Aug;6(4):307–318. doi: 10.1016/0378-1119(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. Studies on the mechanism of replication of adenovirus DNA. V. The location of termini of replication. Virology. 1977 Mar;77(1):149–157. doi: 10.1016/0042-6822(77)90414-7. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Function of adenovirus terminal protein in the initiation of DNA replication. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2221–2225. doi: 10.1073/pnas.79.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate V. E., Philipson L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979 Jun 25;6(8):2769–2785. doi: 10.1093/nar/6.8.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Aleström P., Pettersson U. Sequence of inverted terminal repetitions from different adenoviruses: demonstration of conserved sequences and homology between SA7 termini and SV40 DNA. Cell. 1979 Jul;17(3):705–713. doi: 10.1016/0092-8674(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Winters W. D., Russell W. C. Studies on the assembly of adenovirus in vitro. J Gen Virol. 1971 Feb;10(2):181–194. doi: 10.1099/0022-1317-10-2-181. [DOI] [PubMed] [Google Scholar]
- Wright H. T., Nurse K. C., Goldstein D. J. Nalidixic acid, oxolinic acid, and novobiocin inhibit yeast glycyl- and leucyl-transfer RNA synthetases. Science. 1981 Jul 24;213(4506):455–456. doi: 10.1126/science.7017932. [DOI] [PubMed] [Google Scholar]