Abstract
Background
Continued, persistent gambling to recover accumulating losses, or ‘loss-chasing’, is a behavioral pattern linked particularly closely to gambling disorder (GD) but may reflect impaired decision-making processes relevant to drug addictions like cocaine-use disorder (CUD). However, little is known regarding the neurocognitive mechanisms of this complex, maladaptive behavior, particularly in individuals with addictive disorders.
Methods
Seventy participants (25 GD, 18 CUD, and 27 healthy comparison (HC)) completed a loss-chase task during fMRI. Engagement of functional brain networks in response to losing outcomes and during decision-making periods preceding choices to loss-chase or to quit chasing losses were investigated using independent component analysis (ICA). An exploratory factor analysis was performed to examine patterns of coordinated engagement across identified networks.
Results
In GD relative to HC and CUD participants, choices to quit chasing were associated with greater engagement of a medial frontal executive-processing network. By comparison, CUD participants exhibited altered engagement of a striato-amygdala motivational network in response to losing outcomes as compared to HC, and during decision-making as compared to GD. Several other networks were differentially engaged during loss-chase relative to quit-chasing choices, but did not differ across participant groups. Exploratory factor analysis identified a system of coordinated activity across prefrontal executive-control networks that was greater in GD and CUD relative to HC participants and was associated with increased chasing persistence across all participants.
Conclusions
Results provide evidence of shared and distinct neurobiological mechanisms in substance and behavioral addictions, and lend insight into potential cognitive interventions targeting loss-chasing behavior in GD.
Keywords: addiction, cocaine-use disorder, fMRI, gambling disorder, ICA, loss-chasing
1. Introduction
‘Loss-chasing’, or continued gambling in an attempt to recover losses, is a behavioral pattern that is arguably unique to gambling relative to substance addictions (American Psychiatric Association, 2013), and may differentiate the most severely affected disordered gamblers from non-problem gamblers (Breen and Zuckerman, 1999; Corless and Dickerson, 1989; James et al., 2016; O’Connor and Dickerson, 2003; Toce-Gerstein et al., 2003). Loss-chasing has been linked to heightened impulsivity, reward and loss sensitivity, emotional regulation and decision-making (Bibby, 2016; Breen and Zuckerman, 1999; Lister et al., 2016; Ochoa et al., 2013; Parke et al., 2016). While loss-chasing represents as a significant feature of compulsive gambling, reflecting poor self-control and impaired decision-making (el-Guebaly et al., 2012; Robbins and Clark, 2015), the neural mechanisms underlying the behavior in individuals with addictive disorders remain unclear.
Loss-chasing is a salient feature of decision-making under risk and uncertainty even in non-gambling populations (Shafir and Tversky, 1995). Initial neurobiological investigations of loss-chasing behavior in minimally-experienced gamblers suggest contributions of distinct and dissociable neural systems (Campbell-Meiklejohn et al., 2011; Campbell-Meiklejohn et al., 2008). Decisions to discontinue (or ‘quit’) chasing losses is associated with activity in the dorsolateral prefrontal, anterior cingulate, striatum and parietal cortices (Campbell-Meiklejohn et al., 2008), regions that are commonly associated with networks of executive cognitive functioning (Niendam et al., 2012). By comparison, decisions to chase losses are associated with neural activity in ventral prefrontal regions, consistent with impulsive behavior and impaired decision-making (Fineberg et al., 2010; Hare et al., 2009). Furthermore, increased activity in the anterior cingulate following losing outcomes is associated with the subsequent decisions to quit loss-chasing (Campbell-Meiklejohn et al., 2008), suggesting terminating a chase is associated with increased emotional processing and cognitive conflict in response to loss outcomes (Kim et al., 2010). Similarly, dissociable and complementary contributions of serotonergic and dopaminergic mechanisms influence chasing behavior (Campbell-Meiklejohn et al., 2012; Campbell-Meiklejohn et al., 2011; Rogers et al., 2011). Together, these initial findings suggest that a complex of executive-control and impulsivity-related systems involved in decision-making and loss-processing may contribute to loss-chasing behavior in minimally experienced gamblers. In individuals with gambling disorder, and possibly addictive disorders more broadly, alterations in the neural mechanisms of executive control, impulsivity and reward/loss processing (Leeman and Potenza, 2012) may contribute to loss-chasing behavior.
As compared to general linear model (GLM) approaches to fMRI analysis, independent component analysis (ICA) allows examination of distinct, functionally integrated brain networks associated complex cognitive processes (Calhoun and Adali, 2006). ICA has been proposed to have several advantages over GLM, including less susceptibility to functional heterogeneity and the ability to separate inhibitory and excitatory influences on neuronal activity (Xu et al., 2015; Xu et al., 2016). ICA is a data-driven, network-based computational procedure that has been used to identify functional alterations in the multiple brain networks that contribute to cognitive control (Worhunsky et al., 2013), decision-making (Elton et al., 2017), and during resting-state (Ding and Lee, 2013) in individuals with substance-use disorders. Thus, the current study aimed to extend previous investigations of loss-chasing behavior by examining activity in functional brain networks in individuals with gambling disorder (GD), individuals with cocaine-use disorder (CUD) and a healthy comparison (HC) sample. Participants played a modified version of the loss-chase task (Campbell-Meiklejohn et al., 2008) during fMRI. that allows We hypothesized that ICA-identified networks associated with executive function and motivational processing would be functionally related, or ‘engaged’, in response to losing outcomes and during decision-making periods of the loss-chase task. We expected greater engagement of medial frontal and fronto-parietal networks in GD relative to HC during decision-making, and losing outcomes, preceding choices to quit compared to continue loss-chasing. We also expected ventromedial prefrontal and striatal networks would be more engaged in GD relative to HC during decision-making to continue loss-chasing compared to quit-chasing. It was expected that GD and CUD participants would exhibit shared, addiction-related, and distinct, disorder-specific, patterns of network engagement. Finally, we performed an exploratory factor analysis of engagement patterns of ICA-identified networks to examine differences between GD, CUD and HC individuals in coordinated network activity.
2. Methods
2.1. Participants
Participants were 25 individuals with GD, 18 with CUD and 27 HC individuals (Table 1) recruited from the local community. GD and CUD participants were non-treatment-seeking, and all participants were assessed using semi-structured clinical interviews according to DSM-IV criteria (SCID; (First et al., 2002)). Exclusion criteria included the presence or history of psychotic disorder or other serious mental, neurologic or general medical illness that would interfere with the ability to participate in fMRI procedures (e.g., implanted devices, claustrophobia). GD participants were excluded for a co-occurring current substance addiction (other than tobacco/nicotine), and CUD participants with gambling-severity scores indicative of probable problematic gambling (Lesieur and Blume, 1987) were excluded from the current analyses. Urine toxicology screening for cocaine, marijuana (THC), opiates, amphetamine/methamphetamine, methylenedioxymethamphetamine (MDMA), barbiturates, phencyclidine (PCP), and benzodiazepines (Integrated EZ Split Key Cup; Redwood Toxicology Laboratories, Santa Rosa, CA, USA) and alcohol breathalyzer screening (Alco-Sensor III; Intoximeters, Saint Louis, MO, USA) were performed at the time of scanning to confirm no recent substance use in GD and CUD participants. Study procedures were approved by the Yale Human Investigations Committee, and participants provided written informed consent.
Table 1.
Participant characteristics and task performance
| HC | GD | CUD | F/t/χ2 (P) | |
|---|---|---|---|---|
| N | ||||
| Participant characteristics | 27 | 25 | 18 | |
| Gender, Female (%) | 12 (44.4) | 9 (36.0) | 7 (38.9) | 0.40 (0.82) |
| Age, years (SD) | 33.6 (10.8) | 38.4 (11.5) | 43.7 (5.3) | 5.48 (0.006) |
| IQ, estimated IQ (SD) | 108 (9.9) | 103.1 (13.2) | 94.7 (9.4) | 7.89 (0.001) |
| Tobacco user, N (%) | 2 (7.4) | 9 (36.0) | 12 (66.7) | 17.37 (<0.001) |
| Disorder chronicity, years (SD) | – | 13.5 (10.0) | 14.4 (7.9) | 0.30 (0.77) |
| Loss-chase performance | ||||
| Chase decision-time, ms (SD) | 1477 (672) | 1482 (674) | 1736 (749) | 0.91 (0.407) |
| Quit decision-time, ms (SD) | 1661 (879) | 1519 (668) | 1727 (463) | 0.49 (0.617) |
| Control reaction time, ms (SD) | 1269 (684) | 1167 (568) | 1390 (487) | 0.73 (0.487) |
| Chase depth (SD) | 1.7 (0.2) | 1.8 (0.2) | 1.8 (0.2) | 1.21 (0.306) |
| Quit depth (SD) | 1.9 (0.5) | 1.7 (0.4) | 1.8 (0.3) | 1.86 (0.164) |
| Chase value, $ (SD) | 230 (48) | 234 (51) | 221 (40) | 0.44 (0.644) |
| Quit value, $ (SD) | 266 (96) | 230 (88) | 256 (49) | 1.24 (0.296) |
| Chases won, per run (SD) | 3.0 (1.4) | 2.9 (1.4) | 2.7 (1.4) | 0.35 (0.707) |
| Maximum losses, per run (SD) | 1.7 (0.7) | 2.2 (1.3) | 1.9 (1.4) | 1.32 (0.273) |
Abbreviations: HC, Healthy comparison; GD gambling disorder; CUD, cocaine-use disorder.
2.2. Loss-Chase Task
Participants completed a modified version of the loss-chase task (Figure 1; (Campbell-Meiklejohn et al., 2008)). Prior to scanning, participants were instructed that they had been given a hypothetical $20,000 endowment to participate in a decision-making task. They were informed that, in groups of 10 consecutive participants, the individual with the largest amount remaining in their endowment would receive $50 in addition to research compensation. Participants completed a brief practice prior to scanning to ensure comprehension of the task structure and progression.
Figure 1.
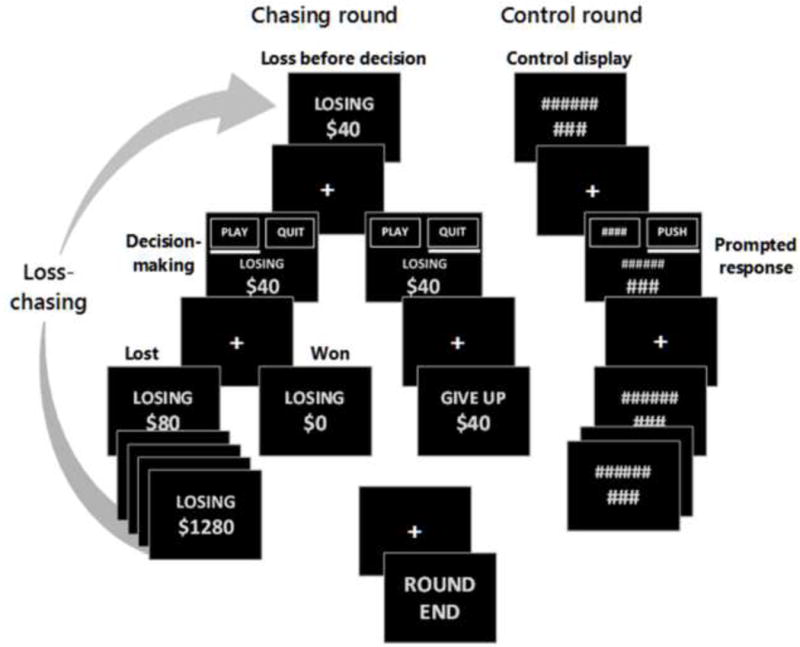
Schematic of the loss-chase game.
The loss-chase task was performed in two consecutive fMRI runs, each consisting of 12 ‘chasing’ rounds and 6 ‘control’ rounds. Chasing rounds began with the imposition of an initial loss ($40, $80, $160 or $320). Participants were given the option to either ‘play’ a double-or-nothing wager (to try to recover the loss) or to ‘quit’ the current round and surrender the loss. Within each chasing round, participants were allowed to continue double-or-nothing wagers (i.e., chase losses) until a maximum loss of $1280 was accrued, a winning outcome (i.e., recovery of accumulated losses) was delivered, or the option to terminate a chase and surrender losses (i.e., quit chasing) was selected. Outcomes of each round were pre-determined (and randomly ordered) such that, if participants elected to chase every loss, half of the rounds would result in the recovery of losses, while the other half would result in a maximum loss. To encourage engagement in both chasing and quitting behavior, participants were informed that solely chasing or quitting was not the optimal strategy; however, they were not provided explicit details regarding the outcome probabilities and performed the task under uncertainty. Control rounds had the same structure as chasing rounds; however, all stimuli were replaced with hash marks to match visual stimuli, and one button was randomly labeled ‘push’ to prompt participants to press the corresponding button to move forward in the control round. Additional details of the loss-chase task are provided in Supplemental Material1.
Loss-chase task performance measures included decision/response times, percentage of decisions to chase losses and the average value of losses associated with decisions to chase and decisions to quit. In addition, the average ‘depth’ of each decision, or the number of sequential decisions within a round, was calculated for all chase and quit decisions. For fMRI analyses, trials were defined as beginning with the delivery of a loss (either imposed or incurred) and ending with the selection to continue or quit chasing. The ‘outcome’ and subsequent ‘decision-making’ period for each trial were modeled separately and classified by the participant’s choice at the end of the trial. That is, chase-related losing outcomes preceded chase-related decision-making periods, which preceded responses to continue chasing. Similarly, quit-related losing outcomes and quit-related decision-making periods preceded responses to quit chasing.
2.3. fMRI Acquisition And Image Processing
Image acquisition was performed on two Siemens Trio 3T systems (Siemens AG, Erlangen, Germany). Identical acquisition procedures and sequences were employed on both systems. Groups did not differ in proportion scanned on each magnet (χ2=2.93, P=0.23), and there was no main effect of magnet (F9,56=0.80, P=0.62) or group-by-magnet interaction (F18,114=1.12, P=0.43) on loss-chase task performance measures. Details of the fMRI acquisition parameters and spatial processing are provided in Supplemental Materials1.
2.4. Independent Component Analysis And Network Selection
ICA was performed on the fMRI time series using the Group ICA of fMRI Toolbox (GIFT v2.0e; http://icatb.sourceforge.net) (Calhoun et al., 2001). An estimate of the number of maximally independent components present in each run was determined using a minimum description length criterion (Li et al., 2007), and the maximum estimate of 78 components across the dataset was used as higher-dimensional extractions improve regional estimates of network integration (Abou-Elseoud et al., 2010). Data from all participants was concatenated into a single group and reduced through a principle component analysis. The 78 components were extracted from the group aggregate using neural network algorithms that maximize network independence (Bell and Sejnowski, 1995). ICA was iterated 20 times using ICASSO to assess stability and consistency of extracted components (Himberg et al., 2004). Component time courses and corresponding spatial source maps were reconstructed and scaled to percent BOLD signal change for each participant (Calhoun et al., 2001). Twenty-four components with stability metrics less than 0.8 or low- to high-frequency spectral ratios less than 3.0 were considered likely artefactual sources (Allen et al., 2011) and excluded from additional analyses.
Task-relatedness of remaining 52 components were assessed using multiple regression analyses of component time courses with the time courses of canonical hemodynamic activity convolved with the six task events: (i) losing outcomes preceding the choice to loss-chase, (ii) losing outcomes preceding the choice to quit chasing, (iii) control outcomes, (iv) decision-making period preceding the choice to loss-chase, (v) decision-making period preceding the choice to quit chasing, and (vi) the period preceding control responses. The limited number of winning and maximum-loss outcomes within runs precluded effective fitting of brain activity to these events; thus, they were not included in the regression. This process produced β-weights that represent a measure of ‘engagement’ or ‘recruitment’ of each component associated with each task event. β-weights of each event for each component were averaged across runs for participants with two runs included in the ICA.
Mixed effects linear models were performed on resulting β-weights to assess the effects of group (GD, CUD, HC), choice (chase, quit) and event (outcome, decision-making) on component engagement in a 3-group × 2-choice × 2-event design using SPSS 22.0 (IBM Corporation, Armonk, NY). Components of interest were determined to be those exhibiting a significant (P<0.05) group-by-choice interaction and/or a main effect of choice on task-related β-weights. One component demonstrated a group-by-choice interaction and a main effect of choice, one component demonstrated a group-by-choice interaction, and six components demonstrated a main effect of choice. The subject-level spatial source maps of the eight components with task-related time courses were then entered into factorial models in SPM12 to determine their regional integration patterns at a voxel-level family-wise error-corrected (FWE) threshold of PFWE<0.001 with an extent threshold of 100 voxels (Figures 2 and 3, Supplemental Table 12).
Figure 2.
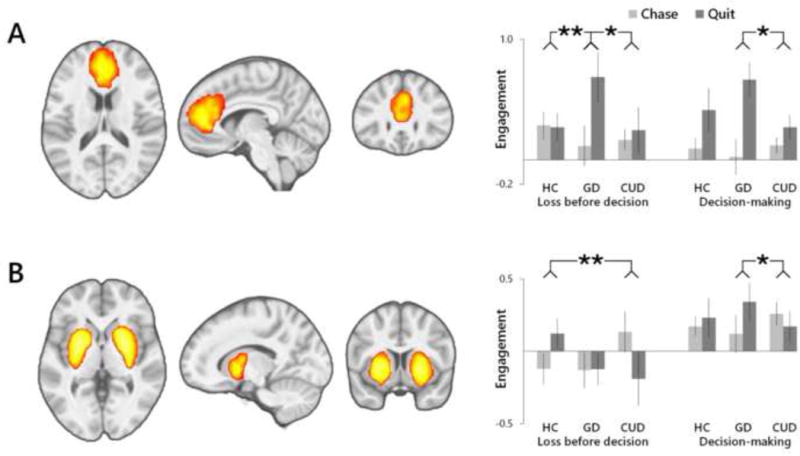
Functional brain networks identified as displaying a group (GD, CUD, HC)-by-choice (chase, quit) interaction in task-related engagement. (A) GD participants exhibited greater quit-relative chase-related engagement of a medial frontal network as compared to CUD and HC following outcomes, and as compared to CUD during decision-making. (B) CUD participants displayed negative quit-relative to chase-related engagement of a striato-amygdala network that differed from HC following outcomes, and differed from greater quit-related engagement in GD during decision-making. Regional integrations displayed at a voxel-level PFWE<0.001, k>100. **P<0.01; *P<0.05.
Figure 3.
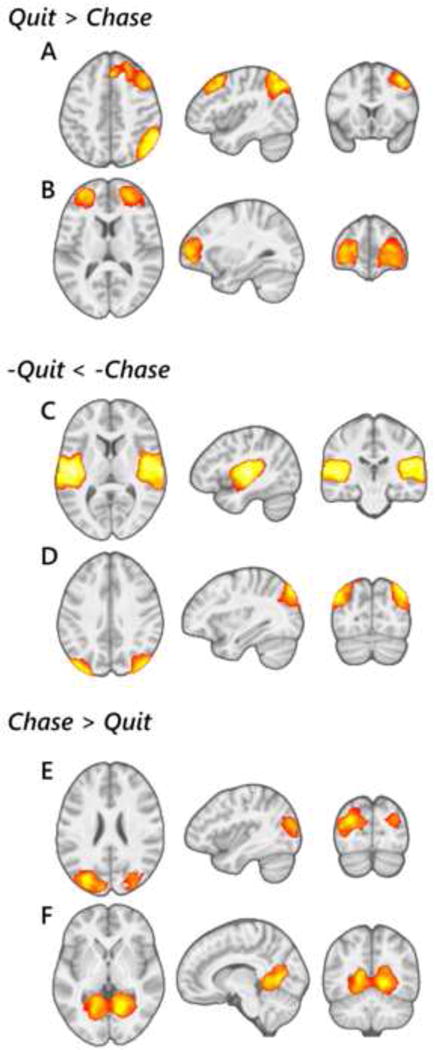
Functional brain networks identified as displaying a main effect of choice (chase, quit) during loss-chase game performance. Right fronto-parietal (A) and middle frontal (B) networks were more strongly engaged during quit- relative to chase-related trials. Auditory (C) and occipito-parietal visual (D) networks were more strongly dis-engaged during quit-relative to chase-related trials. Middle occipital (E) and lingual gyrus (F) visual networks were more strongly engaged during chase-related compared to quit-related trials. Regional integrations displayed at a voxel-level PFWE<0.001, k>100.
Standard linear modeling approaches were used to investigate the nature of task-related effects of interest and explore relationships of component engagement with demographic and clinical measures. Multivariate mixed effects models indicated no between- or within-subjects effects of covariates for acquisition magnet, age, estimated IQ, or smoking status across components of interest (F9,58’s <2.0, P’s>0.05), and thus these variables were excluded from subsequent analyses. There was no main effect of gender or group-by-gender interaction on between- or within-subjects factors across components (F9,56/F18,114’s <1.5, P’s>0.2). Pearson correlations were performed to explore relationships of component engagement with sample characteristics and task performance at a threshold corrected for multiple comparisons (PFDR<0.05).
2.5. Exploratory Factor Analysis
While identified networks represent spatiotemporally independent sources of fMRI BOLD signal, selection procedures based on differential task-related engagement resulted in patterns of highly correlated network activity (Supplemental Table 23). Thus, an exploratory factor analysis was performed to examine potential systems of coordinated engagement across identified networks. Contrast β-weights (i.e., ‘quit minus chase’, for losing outcomes and decision-making periods) for all eight networks were entered into a principal components extraction followed by Varimax rotation. Factors with an eigenvalue >1 were selected, and factor scores for participants were estimated using the Anderson-Rubin method (Anderson and Rubin, 1956) in SPSS 22.0. Items with absolute factor coefficients ≥ 0.4 were considered to load on respective factors, and the reliability of each factor was evaluated using a standardized Cronbach’s alpha measure. Between-group differences in factor scores were examined with exploratory univariate analyses.
3. Results
3.1. Participants And Task Performance
Participant characteristics and loss-chasing performance measures are summarized in Table 1. GD and HC participants did not differ in age or IQ. CUD participants were older than HC participants (t43=3.65, P<0.001), and of a lower IQ than both GD (t41=2.33, P=0.03) and HC (t43=4.53, P<0.001) participants. GD and CUD participants were more likely to be daily tobacco smokers than HC participants (χ2=6.36, P=0.12 and χ2=17.70, P<0.001, respectively). Decisions to quit or chase losses did not differ in duration (F1,69=1.84, P=0.18) and were longer than control responses (F1,69’s>20, P’s<0.001). Participants decided to chase 66.9% (SD=14.0) of all losses, recovering losses on 47.9% (SD=22.9) of possible-win rounds, and incurring the maximum loss on 32.4% (SD=23.9) of non-winnable rounds. That is, participants decided to quit approximately half of the chases that could have resulted in the recovery of losses, and decided to quit approximately two-thirds of chases that would have incurred maximum losses. Participants decided to chase at an average depth of 1.8 decisions (SD=0.2), chasing an average loss of $229 (SD=47). Decisions to quit were made at an average depth of 1.8 decisions (SD=0.4), surrendering an average loss of $250 (SD=83). There were no group effects or pair-wise differences on task performance measures (Table 1).
3.2. ICA-Identified Functional Brain Networks
ICA identified eight distinct, functionally integrated brain networks that demonstrated differential engagement patterns between loss-chasing and quit-chasing trials (Figures 2 and 3, Supplemental Table 14). Identified networks were spatially consistent with intrinsic functional networks associated with executive functioning, motivational mechanisms and sensory processing that have been detected during resting-state and that exhibit task-related engagement patterns (Calhoun et al., 2008; Laird et al., 2011). There were group differences in the engagement patterns of two of the eight networks related to loss-chasing behavior (Figure 2). A medial frontal network (Figure 2A) exhibited a group-by-choice interaction (F2,67=6.33, P=0.003), as well as a main effect of choice (F1,67=20.53, P<0.001) that survived correction for multiple comparisons (PFDR<0.05). This executive-functioning-related network was more strongly associated with events preceding choices to quit relative to chase across participants. In GD, the difference in engagement between outcomes preceding choices to quit relative to chase was greater than in both HC (F1,41=8.49, P=0.005) and CUD (F1,41=4.42, P=0.042). Engagement differences between decision-making periods preceding choices to quit relative to chase were greater in GD as compared to CUD (F1,41=4.72, P=0.036). A striato-amygdala network (Figure 2B) related to motivational mechanisms also demonstrated a group-by-choice interaction (F2,67=4.09, P=0.021) in engagement patterns related to loss-chasing behavior. In CUD, greater engagement in responses to losing outcomes preceding choices to chase relative to quit differed from HC (F1,43=11.11, P=0.002). By comparison, greater engagement in GD during decision-making preceding choices to quit relative to chase differed from CUD (F1,41=4.81, P=0.034).
Six other networks were identified as exhibiting a main effect of choice on task-related engagement across participants (Figure 3). These included greater quit-related relative to chase-related engagement of an executive-function-related right-lateralized fronto-parietal network (Figure 3A) (F1,67=16.17, P<0.001) that survived correcting for multiple comparisons (PFDR<0.05), and in a cognitive-control-related bilateral middle prefrontal network (F1,67=8.14, P=0.005) (Figure 3B). Main effects of choice with greater negative engagement, or greater ‘disengagement’, related to quit as compared to chase choices was exhibited by an auditory-processing-related bilateral temporal network (F1,67=6.22, P=0.015) (Figure 3C) and a visual-processing-related bilateral occipito-parietal network (F1,67=5.47, P=0.022) (Figure 3D). By comparison, additional visual-processing-related networks, including a bilateral middle occipital network (Figure 3E) and a bilateral lingual network Figure 3F), were more engaged during chase-related compared to quit-related choices (F1,67=7.12, P=0.010 and F1,67=5.41, P=0.023 respectively).
There were no correlations between task-related engagement and demographic or loss-chase performance-related measures that survived multiple comparison correction for any of the eight identified networks (PFDR’s>0.05).
3.3. Exploratory Factor Analysis Of Network Engagement
Principal component analysis (PCA) with Varimax rotation was performed to explore systems of coordinated engagement across loss-chase-related networks. Sampling adequacy was validated using the Kaiser-Meyer-Olkin measure (KMO=0.63) and Bartlett’s test of sphericity (χ2=406.2, P<0.001). Five factors with eigenvalues greater than one cumulatively accounted for 66.5% of the total variance (Table 2). Absolute component loadings >0.4 were considered significant in order to determine factor compositions and interpret potential functional systems. The first factor, termed “Sensory Coordination”, consisted of coherent engagement of auditory- and visual-processing-related networks following delivery of outcomes, with inversely coordinated engagement of visual mechanisms and the middle frontal control network during decision-making periods. The second factor, termed “Outcome Sensitivity”, consisted of striato-amygdala engagement following outcomes, and inversely coordinated middle frontal and visual engagement during decision-making. The third factor, “Executive Decisions”, consisted of coordinated engagement of executive control networks and disengagement of sensory networks during decision-making. The fourth factor, “Sustained Control”, consisted of coordinated engagement of executive control networks following outcomes and during decision-making. The last factor, termed “Motivated Decisions”, consisted of coordinated engagement of the striato-amygdala and a visual network during decision-making. Exploratory between-group analysis revealed a main effect of group on Factor 4 (Sustained Control) scores (F2,69=3.51, P=0.036), with GD and CD participants demonstrating greater factor scores than HC participants (Table 2). There were no other group differences in factor scores. Factor 4 scores were significantly correlated with average chase-depth across all participants (r=0.39, P<0.001), and within the HC group (r=0.48, P=0.012) (Supplemental Figure 15).
Table 2.
Exploratory factor analysis of network engagement
| Factor 1: “Sensory Coordination” | Factor 2: “Outcome Sensitivity” | Factor 3: “Executive Decisions” | Factor 4: “Sustained Control” | Factor 5: “Motivated Decisions” | |
|---|---|---|---|---|---|
| Factor loadings | |||||
| Outcome (before Quit > before Chase) | |||||
| Medial frontal | 0.07 | −0.01 | 0.08 | 0.68 | −0.12 |
| Right fronto-parietal | 0.11 | 0.11 | −0.07 | 0.77 | 0.06 |
| Middle-frontal | −0.64 | −0.11 | 0.16 | 0.40 | 0.08 |
| Striato-amygdala | −0.22 | −0.59 | 0.11 | −0.10 | −0.05 |
| Auditory | 0.77 | −0.12 | 0.01 | 0.31 | −0.30 |
| Lingual gyrus (visual) | 0.74 | 0.02 | 0.04 | 0.30 | −0.27 |
| Occipito-parietal (visual) | 0.87 | 0.00 | 0.02 | 0.04 | 0.19 |
| Middle-occipital (visual) | 0.80 | 0.12 | 0.00 | 0.03 | 0.17 |
| Decision-making (Quit > Chase) | |||||
| Medial frontal | 0.27 | −0.31 | 0.67 | 0.18 | 0.08 |
| Right fronto-parietal | 0.01 | 0.39 | 0.58 | 0.46 | 0.12 |
| Middle-frontal | 0.08 | −0.79 | 0.16 | 0.18 | −0.09 |
| Striato-amygdala | −0.20 | −0.03 | 0.00 | 0.01 | 0.83 |
| Auditory | 0.06 | 0.14 | −0.80 | 0.09 | 0.11 |
| Lingual gyrus (visual) | 0.46 | 0.18 | −0.62 | 0.08 | 0.21 |
| Occipito-parietal (visual) | 0.40 | 0.21 | −0.16 | −0.10 | 0.65 |
| Middle-occipital (visual) | 0.00 | 0.81 | −0.06 | 0.18 | −0.02 |
| PCA metrics | |||||
| % Variance | 21.9 | 12.7 | 11.9 | 10.9 | 9.1 |
| Cronbach’s α | 0.83 | 0.64 | 0.63 | 0.51 | 0.37 |
| Group factor scores, (SD) | |||||
| Healthy comparison | 0.07 (0.96) | −0.09 (0.59) | 0.04 (1.04) | −0.38 (1.03) | 0.08 (1.04) |
| Gambling disorder | −0.06 (1.14) | −0.17 (1.45) | 0.12 (1.14) | 0.27 (0.99) | 0.12 (0.81) |
| Cocaine-use disorder | −0.01 (0.89) | 0.36 (0.58) | −0.24 (0.69) | 0.21 (0.83) | −0.28 (1.16) |
| Group effect, F (P) | 0.10 (0.90) | 1.65 (0.20) | 0.71 (0.49) | 3.51 (0.04) | 0.95 (0.39) |
4. Discussion
Functionally integrated brain networks associated with loss-chasing behavior in GD, CUD and HC participants were investigated using ICA. Multiple brain networks were differentially engaged in response to losing outcomes and during decision-making periods preceding choices to quit chasing as compared to choices to continue loss-chasing. These networks included functional circuitry associated with executive-integration and motivational mechanisms that differed between GD, CUD and HC participants, and cognitive and sensory processing networks that were similarly engaged across groups. Exploratory factor analysis further identified systems of coordinated network engagement. A system of executive-function-related networks with coordinated engagement in making choices to quit chasing was greater in individuals with addictive disorders relative to HC.
4.1. Functional Networks Associated With Loss-Chasing
ICA identified multiple, distinct brain networks of functionally integrated activity associated with loss-chasing behavior. The previous investigation into neural correlates of loss-chasing indicated greater activity in prefrontal regions, particularly the anterior cingulate, dorsolateral prefrontal and parietal cortices, as well as the striatum, in making decisions to quit relative to continue chasing in minimally experienced gamblers (Campbell-Meiklejohn et al., 2008). Consistent with these initial results using standard linear (GLM) analysis of BOLD signal, networks encompassing regions of the medial frontal, dorsolateral prefrontal and parietal cortices, and limbic structures were more strongly associated with choices to quit compared to continue loss-chasing.
The identified medial frontal network (Figure 2A) is spatially consistent with a functional system associated with executive integration of cognitive, emotional, reward and response processing (Margulies et al., 2007; Zarr and Brown, 2016). Greater engagement of this network in GD participants preceding choices to quit suggests an increased demand on cognitive processing mechanisms in ceasing a loss-chase and surrendering a loss. This pattern of engagement suggests individuals with GD can successfully inhibit loss-chasing tendencies, rather than exhibiting a loss of control under increased stress (Ciccarelli et al., 2017; Goudriaan et al., 2014; Parke et al., 2016). However, this heightened medial frontal network engagement does suggest that chase-quitting may involve substantial emotional and cognitive control in GD individuals, not present in non-gambling-related response inhibition (de Ruiter et al., 2012), and represents a potential target for cognitive interventions for individuals with GD.
Consistent with the previous investigation (Campbell-Meiklejohn et al., 2008), individuals with gambling experience exhibited greater engagement of a striato-amygdala network (Figure 2B) in making choices to quit relative to continue chasing losses. The identified striato-amygdala network encompasses regions implicated in motivational and reinforcement processing relevant to addictive behavior (Noël et al., 2013; Zorrilla and Koob, 2013). As a distinct intrinsic network, it has been linked to reward and emotion processing and response inhibition (Laird et al., 2011), and functional alterations in these regions, across multiple cognitive domains, are well-established in individuals with addictive disorders (Potenza, 2008). Within the context of loss-chasing behavior, the striato-amygdala network likely reflects a complex of emotional, reward-based, decision-making and impulsivity/compulsivity-related mechanisms (Rutledge et al., 2010; Tom et al., 2007; Watanabe et al., 2013; Yin and Knowlton, 2006). GD as compared to CUD participants displayed greater striato-amygdala engagement during decisions to quit relative to continued chasing, consistent with evidence of increased regional activity during value-based decision-making in individuals with GD (Peters et al., 2013). In CUD, greater engagement associated with choices to loss-chase may be associated with alterations in reward-processing in individuals with substance addictions (Tanabe et al., 2013). The dissociation of engagement patterns related to loss-chasing between individuals with GD and CUD may represent a notable cognitive distinction between substance and behavioral addictions, and require further research to clarify possible underlying cognitive correlates.
The right-lateralized fronto-parietal network (Figure 3A), which is associated with executive functioning domains including response inhibition, attention and decision-making (Laird et al., 2011), did not differ between groups. Neuropsychological studies have indicated impairments in executive functioning processes in individuals with GD (van Holst et al., 2010). However, neurobiological evidence for altered fronto-parietal functioning in GD is limited. Individuals with GD exhibit fronto-parietal hyper-activation under the context of extended delayed rewards (Miedl et al., 2015). However additional research examining non-reward-related cognitive control (Potenza et al., 2003) and reversal learning (Verdejo-Garcia et al., 2015) report no functional difference in fronto-parietal circuitry in GD relative to non-addicted control participants. In individuals with CUD, fronto-parietal hyper-activation (Mayer et al., 2013), hypo-activation (Barrós-Loscertales et al., 2011) and no functional alterations have been reported (Worhunsky et al., 2013). The absence of group differences in fronto-parietal engagement during the loss-chase task suggests general coordination of cognitive resources and attention mechanisms were similarly engaged and intact across participants.
Contrary to hypotheses and the previous report of increased ventromedial prefrontal activity associated with loss-chasing (Campbell-Meiklejohn et al., 2008), no networks involving the ventromedial prefrontal cortex were differentially associated with choices to loss-chase or quit chasing. Post-hoc examination of the 52 non-artefactual networks identified by ICA identified one candidate network involving the ventromedial prefrontal cortex (Supplemental Figure 26). While the network was more negatively engaged during quit-related choices as compared to control trials as in the previous report, there were no differences between quit and chase choices across participants.
4.2. Systems Of Loss-Chasing Networks
Investigations into coordinated systems of functional brain networks may provide further insight into the neural mechanisms of complex cognitive processes such as loss-chasing behavior. An exploratory factor analysis of the loss-chase-related functional networks identified five systems of coordinated engagement. These systems included coherent engagement across outcome and decision-making periods of sensory-processing related networks (Factor 1, “Sensory Coordination”), executive-control networks (Factor 4, “Sustained Control”) and a mixture of motivational, sensory, and executive networks (Factor 2, “Outcome Sensitivity”). Additional systems included decision-making-related systems of coordinated executive and sensory networks (Factor 3, “Executive Decisions”) and motivational and sensory networks (Factor 5, “Motivated Decisions”). Greater factor scores in GD and CUD relative to HC of the Sustained Control system suggests coordinated engagement of executive control networks from losing outcome through decision-making is required to quit loss-chasing in individuals with addictive disorders. Greater factor scores were associated with an increased chase depth across participants, and within HC participants. That is, participants requiring greater coordination of executive functioning to quit chasing were more likely to continue a loss-chase, suggesting this greater coordination of executive control may be associated with overcoming impulsive tendencies or deficient decision-making faculties.
4.3. Implications And Future Directions
This study represents the first to examine the neural correlates of loss-chasing behavior in individuals with GD, and as compared to individuals with a substance addiction and HC participants. Results implicate contributions of several functional brain networks in the performance of loss-chase decision-making. Broadly, network engagement patterns suggest coordinated executive-function-related mechanisms following loss delivery and during decision-making are associated with quitting a loss-chase. In GD, this includes increased engagement of conflict-related processes, perhaps indicating a greater degree of ‘conflict tolerance’ in GD during a loss-chase, with quitting a chase being associated with reaching a greater threshold of emotional and cognitive conflict than non-gamblers. By comparison, continuing a loss-chase was associated with engagement of sensory-processing-related networks across participants, perhaps suggesting loss-chasing may represent an automatic or compulsive-like behavior that is more externally rather than internally or cognitively oriented. In CUD, continuing a loss-chase also differentially engaged reward-related circuitry, perhaps implicating alterations in risk sensitivity or reward-based motivations. Future research investigating contextual influences on CUD-related alterations related to chasing behavior (e.g., using drug-related cues) may help clarify if network engagement differences are associated with substance as compared to behavioral addictions, or if the differences are due to the monetary and gambling context of the loss-chase task. Additional future research could include qualitative interviews with GD individuals regarding emotional and cognitive states associated with loss-chasing and chase-quitting behavior, testing the clinical efficacy of cognitive interventions targeting conflict on loss-chasing tendencies, and neurobiological research to understand possible influences of risk-sensitivity and reward-responsivity on motivational mechanisms during loss-chasing behavior.
4.4. Limitations
The current study is limited by relatively small samples that were collected across two magnets. However, multi-site fMRI research indicates inter-magnet effects on overall BOLD signal are negligible in comparison to inter-subject differences, and there were no magnet-related differences on task-relatedness of ICA-identified networks (Gountouna et al., 2010). The higher-dimensional ICA strategy used in the current analysis can provide improved accuracy of regional constitution of functional networks (Abou-Elseoud et al., 2010); however, task-related engagement differences in only two networks (main effect of choice on engagement of the right fronto-parietal and medial frontal networks), survived corrections for the multiple comparisons performed. Similarly, group differences in loadings from the exploratory factor analysis were not corrected for multiple comparisons. Thus, firm conclusions of the study results are cautioned, and replication of the current findings is needed.
CUD participants were not well-matched on age and IQ to the GD or HC participants, though these differences did not alter ICA or behavioral results when included in analyses. On average, participants chose to chase less than two consecutive losses, limiting examination of network engagement differences as the depth of chases increased and losses accumulated. GD participants did not perform differently relative to non-gamblers (HC and CUD) on the loss-chase task. While this facilitated investigation of loss-chasing neural mechanisms between groups, future testing with paradigms that better elicit chasing behavior in problematic gamblers may identify additional neural correlates of this harm-related behavior.
The loss-chase task is a laboratory-simulated scenario of increasing losses, and thus limits the generalizability to real-world gambling scenarios in which actual financial losses can be accrued over extended periods of time. Inter-session loss-chasing, or re-engaging in a gambling behavior days or weeks later to recover losses, may significantly impact an individual’s well-being (Lesieur, 1979; O’Connor and Dickerson, 2003) and represent a behavior of interest that was not assessed in the current study. Furthermore, the task was performed under hypothetical conditions toward the potential for additional compensation, and thus the lack of real-world consequence may have limited the involvement of emotional and motivational processes that can contribute to loss-chasing behavior (Bibby, 2016; Breen and Zuckerman, 1999; Parke et al., 2016). Similarly, laboratory conditions may influence immersion in gambling behavior (Anderson and Brown, 1984), potentially increasing self-awareness, limiting dissociation and discouraging loss-chasing. Winning and maximum-loss outcomes may also have influenced chasing behavior in subsequent rounds; however, these events did not occur with a frequency that would allow effective examination of associated neural mechanisms. The cognitive mechanisms identified in the current study do however provide some insight into the outcome-processing and decision-making mechanisms that contribute to choices to quit chases within gambling sessions, and these findings may have implications for cognitive and behavioral intervention strategies for disordered gambling and addictions more broadly.
5.0 Conclusions
Investigation of functional brain networks associated with loss-chasing behavior identified multiple distinct functional brain networks related to executive, motivational and sensory processing. Individuals with GD displayed greater engagement of a medial frontal cognitive-integration network, and individuals with CUD displayed aberrant engagement of a striato-amygdala motivational network. Exploratory factor analysis of network engagement identified greater scores in GD and CUD relative to HC in a factor comprised of coordinated activity across executive-control networks associated with choices to quit chasing losses. Network engagement patterns are consistent with models of shared and distinct neurobiological mechanisms in substance and behavioral addictions, and lend insight into potential targets for cognitive interventions related to interrupting loss-chasing behavior in GD.
Supplementary Material
Highlights.
Little is known regarding the neural correlates of loss-chasing behavior
Functional networks and patterns of engagement during loss-chasing were examined
Findings include shared and distinct mechanisms in substance-use and behavioral addictions
Acknowledgments
Role of Funding Source
This research was funded in part by NIH grants from NIDA (R01 DA019039, P20 DA027844, K12 DA000167), the Connecticut State Department of Mental Health and Addictions Services, the Connecticut Mental Health Center, an unrestricted research gift from the Mohegan Sun casino, the Yale Gambling Center of Research Excellence Award grant from the National Center for Responsible Gaming, and the National Center on Addiction and Substance Abuse. Funding agencies were not involved in the study design, conduct or communication outside of funding the research proposal.
Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Lundbeck, Ironwood, Shire, INSYS, RiverMend Health, Opiant/Lakelight Therapeutics, and Jazz Pharmaceuticals; has received unrestricted research support from Mohegan Sun Casino and grant support from the National Center for Responsible Gaming and Pfizer pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for legal and gambling entities on issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. Dr. Rogers has held awards from the Responsible Gambling Trust (now GambleAware) and holds a consultancy agreement with Pfizer Inc.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Conflict of Interest
No conflict declared.
Contributors
RDR, MNP and PDW were responsible for the study concept and design. PDW contributed to the acquisition of data and performed image processing and analyses. RDR and MNP assisted with interpretation of findings. PDW drafted the manuscript and RDR and MNP provided critical revision of the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum Brain Mapp. 2010;31:1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5 doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM 5. American Psychiatric Association 2013 [Google Scholar]
- Anderson G, Brown RIF. Real and laboratory gambling, sensation-seeking and arousal. Br J Psychol. 1984;75:401–410. doi: 10.1111/j.2044-8295.1984.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Anderson TW, Rubin H. Statistical inference in factor analysis. Proceedings of the Proceedings of the Third Berkeley Symposium on Mathematical Statistics and Probability 1956;5 [Google Scholar]
- Barrós-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Ávila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res Neuroimag. 2011;194:111–118. doi: 10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bibby PA. Loss-chasing, alexithymia, and impulsivity in a gambling task: Alexithymia as a precursor to loss-chasing behavior when gambling. Front Psychol. 2016;7 doi: 10.3389/fpsyg.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen RB, Zuckerman M. Chasing in gambling behavior: Personality and cognitive determinants. Pers Individ Dif. 1999;27:1097–1111. [Google Scholar]
- Calhoun V, Adali T, Pearlson G, Pekar J. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Unmixing fMRI with independent component analysis. IEEE Eng Med Biol Mag. 2006;25:79–90. doi: 10.1109/memb.2006.1607672. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn D, Simonsen A, Scheel-Krüger J, Wohlert V, Gjerløff T, Frith CD, Rogers RD, Roepstorff A, Møller A. In for a penny, in for a pound: methylphenidate reduces the inhibitory effect of high stakes on persistent risky choice. J Neurosci. 2012;32:13032–13038. doi: 10.1523/JNEUROSCI.0151-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn D, Wakeley J, Herbert V, Cook J, Scollo P, Ray MK, Selvaraj S, Passingham RE, Cowen P, Rogers RD. Serotonin and dopamine play complementary roles in gambling to recover losses. Neuropsychopharmacology. 2011;36:402–410. doi: 10.1038/npp.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD. Knowing when to stop: The brain mechanisms of chasing losses. Biol Psychiatry. 2008;63:293–300. doi: 10.1016/j.biopsych.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Griffiths MD, Nigro G, Cosenza M. Decision making, cognitive distortions and emotional distress: A comparison between pathological gamblers and healthy controls. J Behav Ther Exp Psychiatry. 2017;54:204–210. doi: 10.1016/j.jbtep.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Corless T, Dickerson M. Gamblers’ self- perceptions of the determinants of impaired control. Br J Addict. 1989;84:1527–1537. doi: 10.1111/j.1360-0443.1989.tb03936.x. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Oosterlaan J, Veltman DJ, van den Brink W, Goudriaan AE. Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depend. 2012;121:81–89. doi: 10.1016/j.drugalcdep.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Ding X, Lee SW. Cocaine addiction related reproducible brain regions of abnormal default-mode network functional connectivity: A group ICA study with different model orders. Neurosci Lett. 2013;548:110–114. doi: 10.1016/j.neulet.2013.05.029. [DOI] [PubMed] [Google Scholar]
- el-Guebaly N, Mudry T, Zohar J, Tavares H, Potenza MN. Compulsive features in behavioural addictions: The case of pathological gambling. Addiction. 2012;107:1726–1734. doi: 10.1111/j.1360-0443.2011.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Smith CT, Parrish MH, Boettiger CA. Neural systems underlying individual differences in intertemporal decision-making. J Cogn Neurosci. 2017;29:467–479. doi: 10.1162/jocn_a_01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: A narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Goudriaan AE, Yücel M, van Holst RJ. Getting a grip on problem gambling: What can neuroscience tell us. Front Behav Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gountouna VE, Job DE, McIntosh AM, Moorhead TW, Lymer GK, Whalley HC, Hall J, Waiter GD, Brennan D, McGonigle DJ, Ahearn TS, Cavanagh J, Condon B, Hadley DM, Marshall I, Murray AD, Steele JD, Wardlaw JM, Lawrie SM. Functional Magnetic Resonance Imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. NeuroImage. 2010;49:552–560. doi: 10.1016/j.neuroimage.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- James RJ, O’Malley C, Tunney RJ. Loss of control as a discriminating factor between different latent classes of disordered gambling severity. J Gambl Stud. 2016:1–19. doi: 10.1007/s10899-016-9592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Chung C, Kim J. Multiple cognitive control mechanisms associated with the nature of conflict. Neurosci Lett. 2010;476:156–160. doi: 10.1016/j.neulet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology. 2012;219:469–490. doi: 10.1007/s00213-011-2550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur HR. The compulsive gambler’s spiral of options and involvement. Psychiatry. 1979;42:79–87. doi: 10.1080/00332747.1979.11024008. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28:1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Nower L, Wohl MJ. Gambling goals predict chasing behavior during slot machine play. Addict Behav. 2016;62:129–134. doi: 10.1016/j.addbeh.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Wilcox CE, Teshiba TM, Ling JM, Yang Z. Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task. Drug Alcohol Depend. 2013;133:235–241. doi: 10.1016/j.drugalcdep.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedl SF, Wiswede D, Marco-Pallarés J, Ye Z, Fehr T, Herrmann M, Münte TF. The neural basis of impulsive discounting in pathological gamblers. Brain Imaging Behav. 2015;9:887–898. doi: 10.1007/s11682-015-9352-1. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23:632–638. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor J, Dickerson M. Definition and measurement of chasing in off-course betting and gaming machine play. J Gambl Stud. 2003;19:359–386. doi: 10.1023/a:1026375809186. [DOI] [PubMed] [Google Scholar]
- Ochoa C, Álvarez-Moya EM, Penelo E, Aymami MN, Gómez-Peña M, Fernández-Aranda F, Granero R, Vallejo-Ruiloba J, Menchón JM, Lawrence NS. Decision-making deficits in pathological gambling: The role of executive functions, explicit knowledge and impulsivity in relation to decisions made under ambiguity and risk. Am J Addict. 2013;22:492–499. doi: 10.1111/j.1521-0391.2013.12061.x. [DOI] [PubMed] [Google Scholar]
- Parke A, Harris A, Parke J, Goddard P. Understanding within-session loss-chasing: An experimental investigation of the impact of stake size on cognitive control. J Gambl Stud. 2016:1–15. doi: 10.1007/s10899-015-9570-x. [DOI] [PubMed] [Google Scholar]
- Peters J, Miedl SF, Büchel C. Elevated functional connectivity in a striatal-amygdala circuit in pathological gamblers. PloS one. 2013;8:e74353. doi: 10.1371/journal.pone.0074353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. The neurobiology of pathological gambling and drug addiction: An overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Robbins T, Clark L. Behavioral addictions. Curr Opin Neurobiol. 2015;30:66–72. doi: 10.1016/j.conb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Wielenberg B, Wojtecki L, Elben S, Campbell-Meiklejohn D, Schnitzler A. Deep brain stimulation of the subthalamic nucleus transiently enhances loss-chasing behaviour in patients with Parkinson’s disease. Exp Neurol. 2011;231:181–189. doi: 10.1016/j.expneurol.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Rutledge RB, Dean M, Caplin A, Glimcher PW. Testing the reward prediction error hypothesis with an axiomatic model. J Neurosci. 2010;30:13525–13536. doi: 10.1523/JNEUROSCI.1747-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir E, Tversky A. Decision Making. In: Smith EE, Oscherson DN, editors. Thinking. MIT Press; Cambridge, MA: 1995. pp. 77–100. 1995. [Google Scholar]
- Tanabe J, Reynolds J, Krmpotich T, Claus E, Thompson LL, Du YP, Banich MT. Reduced neural tracking of prediction error in eubstance-dependent individuals. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toce-Gerstein M, Gerstein DR, Volberg RA. A hierarchy of gambling disorders in the community. Addiction. 2003;98:1661–1672. doi: 10.1111/j.1360-0443.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, van den Brink W, Veltman DJ, Goudriaan AE. Why gamblers fail to win: a review of cognitive and neuroimaging findings in pathological gambling. Neurosci Biobehav Rev. 2010;34:87–107. doi: 10.1016/j.neubiorev.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Verdejo-Román J, Albein-Urios N, Martinez-Gonzalez JM, Gutierrez B, Soriano-Mas C. Neural substrates of cognitive flexibility in cocaine and gambling addictions. Br J Psychiatry. 2015;207:158–164. doi: 10.1192/bjp.bp.114.152223. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Sakagami M, Haruno M. Reward prediction error signal enhanced by striatum–amygdala interaction explains the acceleration of probabilistic reward learning by emotion. J Neurosci. 2013;33:4487–4493. doi: 10.1523/JNEUROSCI.3400-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav. 2013;27:477. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Calhoun VD, Worhunsky PD, Xiang H, Li J, Wall JT, Pearlson GD, Potenza MN. Functional network overlap as revealed by fMRI using sICA and its potential relationships with functional heterogeneity, balanced excitation and inhibition, and sparseness of neuron activity. PloS one. 2015;10:e0117029. doi: 10.1371/journal.pone.0117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN, Calhoun VD, Zhang R, Yip SW, Wall JT, Pearlson GD, Worhunsky PD, Garrison KA, Moran JM. Large-scale functional network overlap is a general property of brain functional organization: Reconciling inconsistent fMRI findings from general-linear-model-based analyses. Neurosci Biobehav Rev. 2016;71:83–100. doi: 10.1016/j.neubiorev.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zarr N, Brown JW. Hierarchical error representation in medial prefrontal cortex. NeuroImage. 2016;124:238–247. doi: 10.1016/j.neuroimage.2015.08.063. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Amygdalostriatal projections in the neurocircuitry for motivation: a neuroanatomical thread through the career of Ann Kelley. Neurosci Biobehav Rev. 2013;37:1932–1945. doi: 10.1016/j.neubiorev.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


