Abstract
Mapping the fine-scale neural activity that underlies epilepsy is key to identify potential control targets of this frequently intractable disease. Yet, the detailed in vivo dynamics of seizure progression in cortical microcircuits remain poorly understood. We combine fast two-photon calcium imaging (30-Hz) with LFP recordings to map, cell by cell, the spread of locally induced (4-AP or picrotoxin) seizures in anesthetized and awake mice. Using single-layer and microprism-assisted multi-layer imaging in different cortical areas we uncover reliable recruitment of local neural populations within and across cortical layers, and find layer-specific temporal delays, suggesting an initial supra-granular invasion followed by deep-layer recruitment during lateral seizure spread. Intriguingly, despite consistent progression pathways, successive seizures show pronounced temporal variability that critically depends on GABAergic inhibition. We propose an epilepsy circuit model resembling an elastic meshwork wherein ictal progression faithfully follows preexistent pathways but varies flexibly in time, depending on the local inhibitory restraint.
Keywords: Two-photon, Epilepsy, Seizure, 4AP, Picrotoxin, Calcium, GABA
In Brief
Wenzel et al. map cortical seizure spread at cellular resolution in vivo, and show that seizures spread reliably, with repeated cell-wise and layer-wise recruitment patterns, yet with greatly variable recruitment durations in absolute time. This ‘elasticity’ is controlled by inhibitory interneurons, as local GABAA-R blockade abolishes the phenomenon.
Introduction
Epilepsy represents a wide range of pathological neural network alterations characterized by recurrent episodes of excessive brain activity. Identifying properties of seizure producing networks (“ictal networks”) may enable more efficient seizure control (Baraban and Loscher, 2014; Krook-Magnuson and Soltesz, 2015). Recent advances in mapping ictal network dynamics at the microscale have unveiled unexpected complexity (Bower et al., 2012; Cymerblit-Sabba and Schiller, 2012; Feldt Muldoon et al., 2013; Keller et al., 2010; Truccolo et al., 2014; Truccolo et al., 2011), challenging the classical view of epilepsy as a condition of stereotyped ictal events (Szabo et al., 2015). In fact, the monitoring of the recruitment of neural populations to successive seizures in humans using multi-electrode arrays has led to contrasting conclusions suggesting either strict reproducibility of neuronal spiking patterns (Truccolo et al., 2011), or lack of such reproducibility close to the epileptic focus (“ictal penumbra”, or “propagation area”) (Schevon et al., 2012) or completely non-repeated recruitment patterns (Bower et al., 2012). Part of the reason behind this controversy could be technical: it remains challenging for multi-electrode approaches to disambiguate the activity of densely packed neuronal circuits, especially for cells far from the electrode. Electrical recordings are inherently sparse when compared to the actual neural population density, complicating definitive conclusions. On the other hand, the few in vitro reports employing high-resolution optical imaging of ictal networks had too low temporal resolution to uncover the true spatiotemporal dynamics of ictal networks (Badea et al., 2001; Cammarota et al., 2013; Feldt Muldoon et al., 2013; Lillis et al., 2015; Neubauer et al., 2014; Tashiro et al., 2002; Trevelyan et al., 2007). To this date, there have been only two studies using optical methods to measure the recruitment of epileptic networks at cellular scale in vivo (Baird-Daniel et al., 2017; Muldoon et al., 2015). Baird-Daniel et al. compared the differential recruitment of glial versus neuronal populations to acute seizures at a population scale. Muldoon and colleagues did not map seizures but interictal spikes, and demonstrated heterogeneous recruitment of local microcircuits across seemingly stereotyped local field potential (LFP) events. Thus, despite being of great conceptual and therapeutic interest, the recruitment dynamics of densely packed neural networks during cortical seizure progression has still not been characterized in detail. In addition, it is unknown if there are layer-specific recruitment dynamics during ictal spread in vivo. However, understanding how exactly seizures progress in the living brain may hold critical new clues on how to stop their expansion.
To address these questions, we combine fast 30Hz resonant two-photon calcium imaging with LFP recordings in two mouse models of acute seizures (4-Aminopyridine [4-AP], or picrotoxin [Ptx]) in anesthetized and awake mice, imaging both supra- and infragranular layers in different cortical areas. We find that neural recruitment to ictal activity maintains relative spatiotemporal reliability, even at the single cell level, and that this reliability holds true across cortical areas and layers, with an initial supra-granular cortical invasion closely followed by recruitment of deep layers. However, absolute temporal micro-progression of seizures varies profoundly across events, revealing a constrained ictal network that flexibly stretches and compresses in time. We show that this progression ‘elasticity’ (i.e., relative spatiotemporal reliability despite progression variability in absolute time) critically hinges on the activity of local GABAergic interneurons, as compromising their signaling results in the acceleration and invariance of ictal progression. Thus, cortical circuits, at least during seizures, can propagate activity through the same pathways yet with greatly variable speeds and delays, both of which depend on the strength of the local inhibitory restraint to excessive network activity.
Results
To map seizure propagation, we employed two pharmacological models of acute seizures using local cortical injection of small amounts of either 4-AP (15mM, 500nl [total amount delivered = 7.5 nmol], layer V) or Ptx (10mM, 500nl [total amount delivered = 5nmol, layer V) in mature mouse neocortex. The two drugs generate seizures through different mechanisms. While the potassium channel blocker 4-AP enhances neuronal firing through increased pre-synaptic glutamate release (Morales-Villagran and Tapia, 1996), the disinhibitory compound Ptx acts as GABA-A receptor antagonist (Krishek et al., 1996). The models provided a two-pronged approach for studying the repeated and variant spatiotemporal details of spreading seizures at a fine scale, in a setting where intra- and extrafocal compartments are precisely defined.
Two-photon calcium imaging of neocortical seizure spread in vivo
We were interested in mapping cellular recruitment during spread of ictal activity, and used in vivo two-photon calcium imaging (OGB-1, GCaMP6s or GCaMP6f) to monitor action potential activity in neural populations (Chen et al., 2013; Stosiek et al., 2003; Yuste and Denk, 1995; Yuste and Katz, 1991). For imaging seizure spread during mild anesthesia, a small craniotomy above somatosensory cortex was established (Figure 1A). As a gross indicator of ictal activity within the examined area, we measured the local field potential (LFP) with a sharp glass microelectrode, carefully lowered into the cortex next to the imaged field of view (FOV, Figure 1A). The positioning of the LFP pipette in proximity, yet outside the seizure initiation site did not affect the temporal signature of electrographic seizures, even though LFP signal amplitude decreased with distance to the initiation site (Figure S1 A). For induction of ictal events, a second glass micropipette containing 4-AP was inserted into the cortex to a depth of ~480 μm after baseline imaging, at a distance of ~1.5 – 2 mm to the FOV (Figure 1A and B). No epileptiform activity was induced by saline injection alone (Figure S1 B). Animals’ heart rate, breath rate, and peripheral blood oxygenation were monitored via a paw sensor throughout experiments to insure stable vital parameters (Figure S1 C). Even though leading to hundreds of interictal LFP spikes (IIS), Ptx injections did not lead to full ictal events during anesthesia (data not shown), and were henceforth used only in experiments during wakefulness. In the absence of any chemoconvulsant, local populations in layer II/III (LII/III) displayed ongoing sparse and distributed calcium activity during baseline (Figure 1C left, and 1 E). In contrast, full ictal events post 4-AP entailed sustained firing of large numbers of neurons in the field of view (Figure 1C middle, Figure 1D and F, Figure S1 D, movie S1 and S2). Ictal invasion happened in a continuous wave of intense neuronal firing that slowly propagated across the FOV. During anesthesia, 4-AP induced electrographic seizures and imaged calcium transients corresponded in time (Figure 1D, Figure S1 D), and the rise in calcium was correlated with the typical increase in spectral power of the ictal LFP signal over a wide frequency range (1–100Hz), reflecting massive synaptic barrages during seizures (Figure S1 E). However, individual calcium deflections could only be directly related to lower frequency ictal activity towards the end of seizures (see e.g. movie S2), because even fast calcium indicators like OGB-1 or GCaMP6f have too low temporal kinetics to resolve individual LFP deflections during tonic ictal firing (Khoshkhoo et al., 2017). The propagation velocity of the optical invasion of the field of view was similar to the Jacksonian march described in humans and experimental seizure models where inhibition is intact (7 experiments [exp.] under anesthesia, 71 seizures, 0.64 ± 0.18 mm/sec) (Jasper, 1969; Trevelyan et al., 2007; Wong and Prince, 1990). Notably, the pre-ictal neural activity in yet not invaded areas during pending seizure spread was sparse until the sharp rise in fluorescence upon arrival of the ictal wave front (Figure 1D and F, Figure S1 D). This activity pattern was in sharp contrast to the seizure initiation site, where locally confined, enhanced calcium activity could be imaged almost immediately after 4-AP injection (data not shown). Importantly, our temporal imaging resolution of 30Hz was sufficient to capture individual cell recruitment to ictal activity (Figure 1F and G, Figure S1 D).
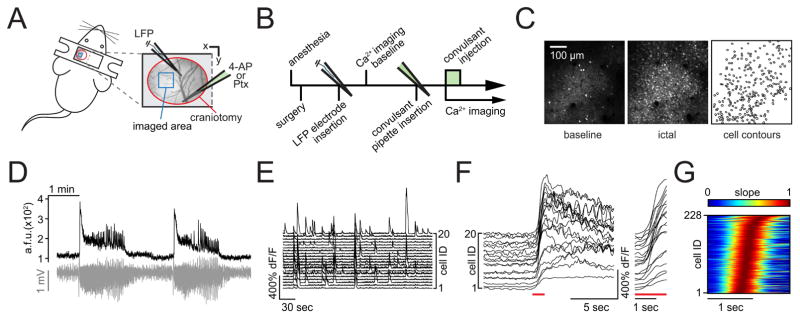
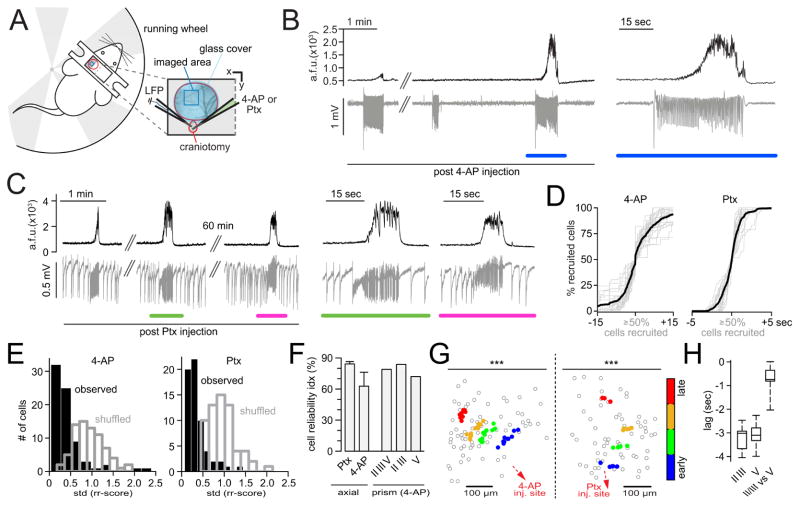
Figure 1. In vivo two-photon calcium imaging of seizure spread with single cell resolution.
(A) Experimental setup with surgical approach over left somatosensory cortex; craniotomy encircled in red, imaged field of view (FOV) within the seizure propagation area in blue; each experiment (‘exp.’) involved the insertion of two glass micropipettes, one (blue) containing a silver chloride electrode for LFP (local field potential) recording, the other (green) containing 4-AP (15 mM, injection vol. 500 nl [total amount delivered = 7.5 nmol]) or Ptx (10 mM, injection vol. 500 nl, [total amound delivered = 5 nmol]). (B) Typical experimental workflow. (C) Propagation area in left somatosensory cortex, representative 3 sec average (avg) fluorescence images of neural activity (GCaMP6s) during baseline (left) and full ictal event (middle, see also movie S 1.1); contour plot of registered cells (right). (D) Avg calcium transient of FOV (black trace, GCaMP6s, imaging depth ~150μm beneath the pial surface) and corresponding LFP (gray trace) post 4-AP. (E) Calcium transients of 20 representative cells within FOV during baseline. (F) The same 20 cells post 4-AP, optical seizure break-in (underlined in red) magnified on the right. (G) Representative example of the optical break-in of the ictal wave. Normalized first derivative of ΔF/F. Cell recruitment to ictal activity ordered in time by maximum slope.
Stereotypical spatiotemporal recruitment of neural populations by seizures
To investigate the characteristics of local network recruitment during seizure spread, we analyzed all neurons in the field of view that showed a visible change in fluorescence upon optical break-in of ictal activity and whose somata could be followed stably across the analyzed time period (n = 7 exp. under anesthesia [OGB-1 = 4, GCaMP6s = 3], total # of seizures = 71, average # of analyzed cells = 201 ± 25 s.e.m.). Corresponding in time to the incoming ictal wave, neurons were recruited in a continuous, sigmoid temporal curve (Figure 2A). First, we mapped temporal population recruitment patterns, specifically examining the regularity of individual cell and sub-region recruitment to seizures. Arranging cell recruitment time points (max. slope of ΔF/F) of an individual seizure revealed that the tilt of the temporal ordering was coarsely preserved across ictal events (Figure 2B). When we tiled the imaged region instead of registering individual cells (Figure S2 A), this inherent temporal structure became apparent even in unsorted data (Figure S2 B).
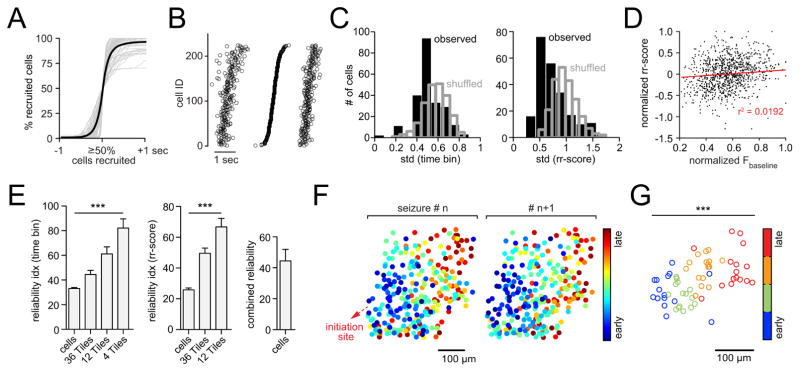
Figure 2. Stereotypical micro-progression of seizures.
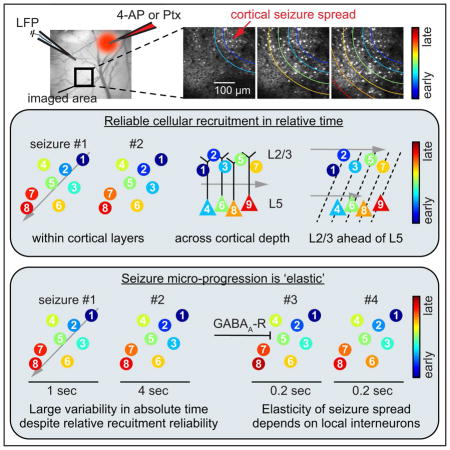
(A) Superposition of all analyzed optical seizure break-ins (gray) centered around the 50% recruitment frame; black graph represents mean temporal recruitment (n = 7 exp., total # of seizures = 71 [11.3 ± 1.358 s.e.m.], total # of cells analyzed = 1402 [201 ± 25 s.e.m.], cell number in % for comparability across exp.). (B) Representative example of 3 consecutive optical seizure break-ins plotted next to each other. The 1st and 3rd event are sorted by the temporal ordering of the 2nd event. Each circle represents an individual cell recruitment time point. (C) Representative exp. with observed (black) versus shuffled (gray) time bin or rr-score standard deviation (std) distributions. (D) Correlogram of cellular baseline fluorescence versus rr-scores. Values are max-normalized for comparability across exp.. The relationship between the two parameters is negligible: 4 exp., 42 seizures, 952 cells. r = 0.1386, r2 = 0.0192. The effect accounts for 1.9% of the variance in the data. (E) Reliability indices displaying the % of cells or spatial sub-regions (tiles) whose temporal recruitment variability (time bin or rr-score std) are < 5 % of all shuffled values of analyzed cells or tiles (p<0.05). Recruitment reliability increased with spatial coarseness (one-way ANOVA, n = 7 exp., total # of cells = 1402, time bin and rr-score classification: p<0.0001). Right: Combined (“reliable” in time bin and/or rr-score classification) cell-wise reliability index. (F) Spatiotemporal maps of cell recruitment in two consecutive seizures indicating grossly preserved relative recruitment. Each dot represents an individual cell. 4-AP injection site (red arrow) is located ~1.5mm posterior to FOV (somatosensory cortex). (G) Same exp., spatial avg coordinates of temporally defined population quartiles (25% earliest recruited cells, 25–50%, 50–75%, 75–100%) across 16 seizures show consistent spatiotemporal propagation (bivariate ANOVA: n=16 seizures, F[3,60] = 11.64, p = 0.000004, all 7 axial exp. under anesthesia p<0.001). Each circle represents the avg coordinate of all cells belonging to the respective quartile.
To quantify and verify this conserved structure at the single cell scale, we used two different analytical approaches. First, regardless of its duration, we divided every ictal break-in into three equally sized time bins (early, intermediate, and late; Figure S2 C, upper panel), calculating a standard deviation (std) of bin-membership for each cell across the entire experiment such that, if a given cell were always recruited to the seizure at the same relative time, its std would equal zero. We additionally established a more continuous method by classifying a cell’s recruitment time relative to the entire population (similarly to a z-score), separately for each seizure (relative recruitment score [rr-score], Figure S2 C, lower panel). The observed time bin or rr-score distributions for each cell and their std were compared to randomized surrogate distributions derived from a temporal reshuffling procedure (Figure S2 D). Both methods revealed left-shifted std distributions, indicating a smaller relative onset-time variability in the observed data set than would be expected by chance given population recruitment (Figure 2C). To exclude the possibility that these results were biased by the cells’ indicator load (e.g. the higher the load the sooner the measured recruitment), we performed a correlation analysis between cellular baseline fluorescence and the corresponding recruitment, which showed no systematic relationship (Figure 2D, all 4 OGB-1 exp., 42 seizures, 952 cells. r = 0.1386, r2 = 0.0192. The effect accounts for 1.9% of the variance in the data). After obtaining cellular time bin and rr-score std’s, we generated a reliability index for individual cells that were defined as “reliable” when their std was smaller than 95% (p<0.05) of those in the randomized dataset. Again, both approaches revealed a substantial percentage of temporally reliably recruited neurons (Figure 2E, “cells”). This reliability further increased with growing spatial coarseness (Figure 2E, “tiles”). It is noteworthy that reliability indices of time binning and rr-score classification were not necessarily built of the same cells, but in fact had non-overlapping sections. The combined reliability index, where a cell was called “reliable” if reliable in at least one of the two methods (time bin or rr-score), was higher than in either individual classification (Figure 2E, right).
The fact that cell and tile analysis showed stable relative recruitment ordering to subsequent seizures implied that seizures propagate in a spatially organized manner, and indeed, we found a coarsely maintained spatial pattern of cell recruitment (Figure 2F). To quantify this effect, we implemented a 2-dimensional ANOVA approach by categorizing cells based only on their recruitment times (i.e. temporal quartiles). We compared i) the distance of individual cells to the spatial mean of their respective quartile to ii) the distance of all cells to the spatial mean of the entire analyzed population (Figure 2G, same exp. as in 2 F; Figure S2 E). This approach yielded significant bivariate F-values for all seven experiments (F-values: F[3,48] = 11.595, F[3,28] = 33.260, F[3,36] = 11.251, F[3,52] = 22.208, F[3,36] = 12.67, F[3,60] = 11.64, F[3,8] = 41.224; all p-values < 0.001).
Stereotypical local population recruitment patterns across cortical layers
To test whether network reliability holds true across cortical layers, we employed a recently introduced technique enabling multilayer imaging through implanted glass microprisms (1×1×1mm, Figure 3A) (Andermann et al., 2013). Again, a sharp glass microelectrode was carefully lowered into the cortex close by the prism face for LFP recordings (Figure 3A), and a second sharp glass micropipette containing 4-AP was advanced into the cortex to a depth of ~480 μm, at a distance of ~1.5 – 2 mm to the implanted prism. Imaging was carried out at a distance of 180–300 μm from the vertical prism face, where no damage of the cortex is to be expected due to prism insertion (Chia and Levene, 2009b). In accordance with previous reports (Andermann et al., 2013; Chia and Levene, 2009b), visualized vasculature and cell anatomy appeared physiological and local network activity showed sparse cell firing (Figure 3B, movie S3), as observed during axial imaging of superficial layers (LII/III) during baseline. Correct vertical positioning of the prism was insured by visualizing the full extent of layer V (LV) pyramidal neurons with their apical dendrites extending across LIV into LII/III. As observed in axial plane LII/III experiments, seizures invaded the imaged translaminar FOV in a continuous fashion (Figure S3 B, movie S4 and S5). Side by side plotting of individual cells’ recruitment time points of consecutive seizures showed that the earlier observed coarse preservation of temporal recruitment within LII/III holds true across cortical depth (Figure 3C). Likewise, quantification of multilayer cell recruitment during lateral seizure spread revealed left-shifted std distributions of individual cell bin-memberships or rr-scores (Figure 3D). Consequently, a substantial percentage of neurons in LII/III and LV were shown to occupy reliable relative temporal recruitment positions across seizures (Figure 3E). Again, spatiotemporal onset maps of successive seizures showed a maintained spatial pattern of relative cell recruitment (Figure 3F and G). These experiments showed that network recruitment reliability can be found across the cortical column.
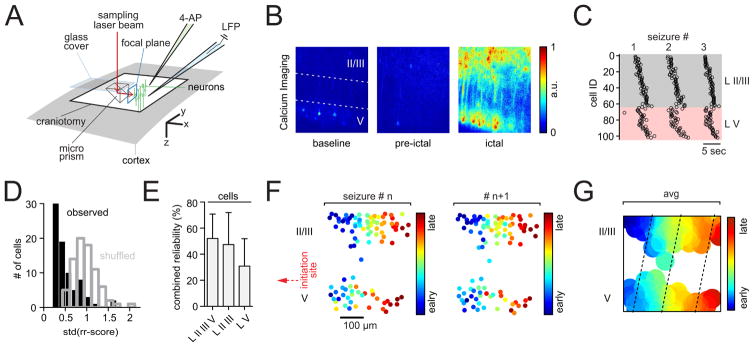
Figure 3. Stereotypical and seizure propagation across cortical layers.
(A) Experimental setup involving cortical microprism implant; brain surface in gray, craniotomy encircled in black, 90° laser beam (red) deflection results in a vertical FOV; neurons in green; again, each exp. involved the insertion of two glass pipettes (LFP [blue], 4-AP [green]). (B) Representative 3 sec avg fluorescence images of multilayer neural activity, recorded under anesthesia in somatosensory cortex during baseline (left, II/III or V = layer II/III or V), directly prior to the seizure break-in (middle), and full ictal event (right, see also movies S3, S4, S5). The injection site of 4-AP was located ~2 mm anterolateral to the prism (in LV), aligned to the edge of the prism face. (C) Representative example of 3 consecutive optical seizure break-ins plotted next to each other, indicating consistent recruitment across cortical layers. Each circle represents an individual cell recruitment time point. (D) Representative exp., observed (black) versus shuffled (blue) rr-score std distribution. (E) Combined reliability index displaying the percentage of cells whose recruitment std are < 5 % of all shuffled std of all analyzed cells (p<0.05, n = 4 exp., total # of seizures = 32 [8 ± 3.6 s.e.m.], total # of cells analyzed = 334 [84 ± 13 s.e.m.]). (F) Spatiotemporal maps of multilayer cell recruitment in two consecutive seizures, indicating preserved cell recruitment. 4-AP injection site (red arrow) is located ~1.5 mm away from FOV. (G) Same exp., avg contour plot of 8 seizures. Note how LII/III appears to be recruited ahead of corresponding LV (dotted lines).
Spreading ictal activity recruits superficial cortex ahead of deep cortical layers
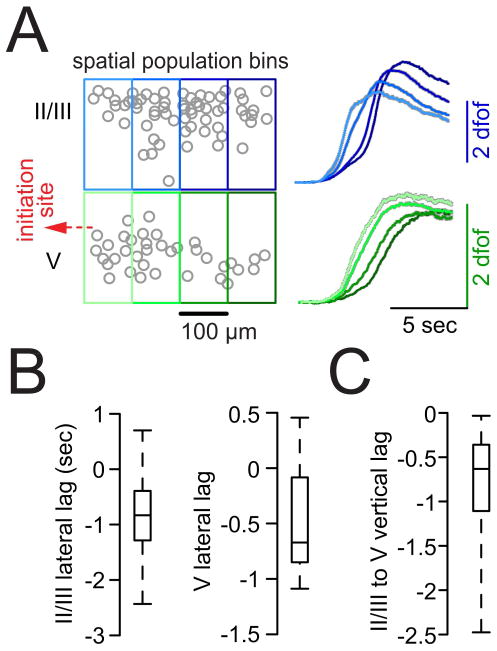
During multilayer imaging, we noticed that relative temporal recruitment of local cell populations seemed to differ across layers (Figure 3G). To quantify layer-specific spatiotemporal recruitment delays, we located groups of neighboring neurons within spatial bins (or “tiles”) of 100 μm width in LII/III and LV and derived an average calcium population transient for every tile, for each electrographic seizure onset of an experiment (Figure 4A, S3 C and S3 D). In accordance with our results from axial imaging in LII/III (Figure 2F and G), tiles in LII/III being located more proximally to the seizure initiation site were consistently recruited earlier than those being situated further distant to it (Figure 4A and S3 C, blue traces). The same spatiotemporal pattern of progression was observed in LV (Figure 4A and S3 C, green traces). To our surprise, and in contrast to previous reports in disinhibited brain slices where LV led lateral spread of epileptiform activity (Telfeian and Connors, 1998), we observed an ictal recruitment of LII/III ahead of LV (Figure 4A and S3). In fact, this bi-directional progression delay was already apparent to the naked eye when inspecting the raw data (movie S4 and S5). To compare layer-specific delays across experiments, we calculated the recruitment time lags of adjacent tile pairs (lateral pairs: tile[prox] - tile[dist]; vertical pairs: tile[II/III] - tile[V]) for every seizure break-in (Figure S3 D). In doing so, we did not only find significant layer-specific average negative time lags of proximal versus distal tiles in nearly all seizures, but also proved a temporally variable yet consistent delay of local LV populations to their LII/III tile counterparts (Figure 4B and C). In sum, we found that cortical seizure spread in the intact brain involves lateral expansion within superficial and deep layers. Intriguingly, we reveal a vertical delay during lateral seizure spread, with a lead of LII/III recruitment over LV.
Figure 4. Supragranular layers are systematically recruited ahead of deep layers.
(A) Left: Paradigmatic multilayer contour plot (LII/III and LV, circles represent individual cells). Small local cell populations were grouped together in spatial bins (tiles) of 100 μm width to assess lateral delays of adjacent tiles and vertical delays of tiles situated above each other. Right: Population avg calcium transients of individual tiles within LII/III or LV across all seizures in one exp. (right, gray shades represent s.e.m., # of seizures = 11, # of analyzed cells = 102, # of spatial tiles = 8). Note that lateral but also vertical tile delays can be appreciated by eye. (B–C) Box plots of lateral (tile[prox]-tile[dist]) or vertical (tile[LII/III]-tile[LV]) recrutiment time lags of adjacent tiles (see also Figure S3 D and SI); boxes represent 25%ile to 75%ile, bands inside boxes display the median recruitment time lag (n = 4 exp., total # of seizures = 32 [8 ± 3.6 s.e.m.]). In nearly all seizures proximal tiles (lateral lag) were recruited prior to their adjacent distal tiles (for lateral lags LII/III and LV: Chi-Square test X2(1) = 12.7, p<0.001); in all seizures LII/III tiles were recruited prior to their corresponding LV tiles (X2(1) = 21.3, p<0.001).
Mapping cortical seizure micro-progression in awake mice
Major advancements in epilepsy research have been achieved by measurements in anesthetized animals. Yet inherently, anesthesia alters neuronal network activity, and it does so in a non-uniform way (Adesnik et al., 2012). Therefore, we set out to investigate whether our findings hold true in the awake condition, using either local 4-AP or Ptx injection. Again, we recorded calcium activity and LFP, in head-restrained yet otherwise freely moving mice, positioned on a running wheel that can tilt flexibly and therefore serve as a suspension during seizure-related motor symptoms. To maximize focal plane imaging stability in the awake state, a thin glass cover slip was positioned over the craniotomy, with a small opening established posterior to it for access of both the LFP, and 4-AP or Ptx pipettes (Figure 5A). Prior to the actual experiment, all animals were habituated to the experimenter and the experimental environment. Like in anesthetized mice, a series of full electrographic, but now also behaviorally detectable seizures including orofacial and limb related motor symptoms and at times alternatingly bilateral convulsions occurred post local 4-AP or Ptx injection (Figure 5B and C). In spite of this, our local injection approach allowed for the study of spreading seizures under stable imaging conditions, since ictal recruitment of imaged cortical areas sufficiently often preceded seizure spread to motor cortices. In comparison to 4-AP, Ptx induced seizures were generally short (4-AP: 71 ± 7.1 sec [5 exp., 26 seizures], Ptx: 19.1 ± 1.34 sec [3 exp., 19 seizures]). Additionally, Ptx injection commonly led to multitudes of IIS in the LFP, which however did not optically recruit the imaged FOV (Figure 5C). Interestingly, and consistently in awake mice, interictal periods and seizure durations displayed a rather irregular pattern. At times, ictal activity failed to invade or only incompletely penetrated the imaged territory (Figure 5B left, Figure 5C left), and there was a consistent delay between the electrographic seizure onset and optical break-in, on the scale of seconds (Figure 5B and C, magnified portions) (Khoshkhoo et al., 2017; Martinet et al., 2015). This observation could be explained by a much slower temporal seizure progression imaged during wakefulness as compared to the anesthetized condition (4-AP: 0.0215 ± 0.003 mm/sec [5exp., awake] versus 0.64 ± 0.18 mm/sec [7exp., anesthetized]). Both the lack of optical penetration of IIS and the persistence of seconds of delay of optical break-in upon electrographic seizure onset present also in the Ptx model, over the duration of an entire experiment (Figure 5C, magnified portions), further supported that the injected chemoconvulsant remained local within the seizure initiation site and did not diffuse into the FOV.
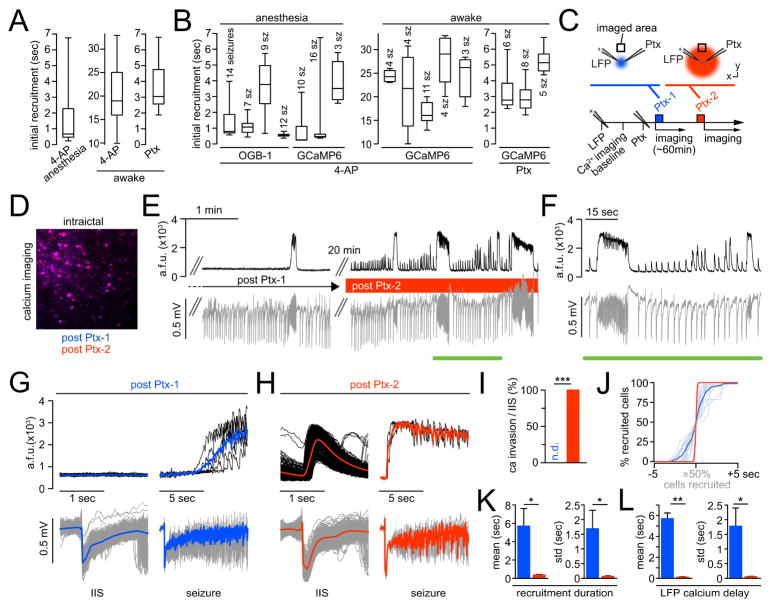
Figure 5. Stereotypical and elastic seizure propagation in awake mice.
(A) Adapted experimental setup; craniotomy outlined in red, FOV in dark blue; glass cover slip in light blue. Two inserted glass pipettes (LFP [blue], 4-AP or Ptx [green, 4-AP: 500 nl, total amount delivered = 7.5 nmol; Ptx: 10mM, 500 nl, total amount delivered = 5 nmol]) (B) Avg population calcium transient (black trace, Thy-1::GCaMP6f, LII/III) and corresponding LFP (gray trace) post 4-AP (~2mm posterior to FOV in somatosensory cortex). Blue underscore marks magnified inset on the right (C) Avg population calcium transient (black trace, Thy-1::GCaMP6f, LII/III) and corresponding LFP (gray trace) post Ptx (~1.5mm posterior to FOV in somatosensory cortex). Green and magenta mark magnified insets on the right. Note the stable lack of calcium responses to IIS in the LFP and slow seizure invasion of the FOV post Ptx. (D) Superposition of all axially imaged seizures (gray) in awake mice, post 4-AP or Ptx, centered around the 50% recruitment frame; black graphs represent mean temporal recruitment (4-AP: n = 5 exp., total # of seizures = 26, total # of cells analyzed = 359 [72 ± 8 s.e.m.], Ptx: n = 3 exp., total # of seizures = 19, total # of cells analyzed = 182 [61 ± 1 s.e.m.]); cell number in % for comparability across exp.. (E) Representative exp. (4-AP or Ptx) with observed (black) versus shuffled (gray) rr-score std distributions. Both seizure models show left-ward shifts of the observed versus shuffled data. (F) Cellular reliability indices (rr-score) across seizure models. Ptx (n = 3 exp., axial imaging), 4-AP (n = 5 exp. axial imaging, 1 exp. multilayer imaging). (G) Representative exp. (4-AP or Ptx): spatial avg coordinates of temporally defined population quartiles (colored dots), plotted within the imaged population (gray circles represent individual cells). Left: 4-AP (bivariate ANOVA: n=11 seizures, F[3,40] = 18.44, p = 1×10−7, all 5 exp. in awake mice with 4-AP p<0.001). Right: Ptx (bivariate ANOVA: n=4 seizures, F[3,12] = 52.87, p = 1.5×10−8, all 3 exp. in awake mice with Ptx p<0.001) (H) Multilayer imaging. Box plots of lateral or vertical onset time lags of adjacent tiles (please see also Figure S3 D); boxes represent 25%ile to 75%ile, bands inside boxes display the median recruitment time lag (1 exp., 4-AP, total # of seizures = 7).
During wakefulness, in line with our anesthetized recordings, neurons in the propagation area were recruited in a continuous, sigmoid temporal curve independently of the employed seizure model, yet over stretched time courses (Figure 5D, movie S6). Cellular recruitment reliability to ictal activity persisted in awake mice, and appeared to be even more pronounced than during anesthesia. In both 4-AP and Ptx induced seizures, we found a clear left-ward shift of rr-score std distributions in the observed versus randomized data (Figure 5E), and consequently obtained high cellular reliability indices, within and across layers (Figure 5F, 4-AP: 5 axial exp., 1 prism; Ptx: 3 exp.). Again, we excluded the possibility that these results were biased by the cells’ indicator expression levels through a correlation analysis between cellular baseline fluorescence and the corresponding recruitment time (Figure S3 E, transgenic GCaMP6f, 5 axial exp. [4-AP], 26 seizures, 360 cells. r = 0.1675, r2 = 0.0281. The effect accounts for 2.8% of the variance in the data). In agreement with our experiments in anesthetized mice, temporal population recruitment quartiles clustered into discriminable spatial domains (Figure 5G, 4-AP: 11 seizures, F[3,40] = 18.44, p = 1.145×10−7; Ptx: 4 seizures, F[3,12] = 52.87, p = 1.591×10−8, other 4-AP exp.: F[3,12] = 138.48, F[3,8] = 15.89, F[3,12] = 32.19, F[3,20] = 56.5; other Ptx exp.: F[3,20] = 447.98, F[3,28] = 91.22; all p<0.001). Finally, layer-specific recruitment delays were also present in seizure spread during wakefulness. As described (Figure 4, Figure S3), we found significant layer-specific average negative time lags of proximal versus distal tiles in all seven seizures imaged through a microprism, and consistent delays of local LV populations to their LII/III respective group of cells (Figure 5H). It is noteworthy that, while lateral time lags were prolonged during wakefulness, trans-laminar lags remained on a par with the anesthetized condition. In conclusion, while differences in temporal seizure progression were found in awake versus anesthetized mice, relative ictal recruitment reliability of local neural networks, spatiotemporally ordered micro-progression, and layer-specific recruitment lags to spreading seizures were also present in experiments during wakefulness.
Seizure micro-progression is elastic
While evaluating the spatiotemporal characteristics of local network recruitment by seizures in both the anesthetized and awake condition, we noticed that, despite the observed relative spatiotemporal stereotypy, the absolute ictal network recruitment varied profoundly in time. To further investigate this phenomenon, we defined the recruitment duration for each recorded seizure as the time period from the first to the last cell recruitment frame, excluding the 5% most deviant cells to the median recruitment frame to minimize outlier-related duration distortions. Across employed seizure models, population recruitment varied from hundreds of milliseconds to several seconds (Figure 6A). Strikingly, this variability was present within individual experiments, too (Figure 6B, 7 exp. [anesthesia], 8 exp. [wakefulness]). Importantly, this phenomenon was not simply explained by a facilitating temporal compression over time, since successive seizures showed well maintained relative spatiotemporal recruitment while their absolute time courses of recruitment compressed and stretched greatly (Figure S4 A). While we could not find a systematic correlational relationship between electrographic seizure length and local population recruitment duration (Figure S4 B), as has been previously described for macroscale ictal recruitment (Martinet et al., 2015), total seizure duration itself displayed temporal variability, too (Figure S4 C). These results unveiled that neural recruitment to spreading seizures displays ‘elastic’ features, that is, relative spatiotemporal reliability despite progression variability in absolute time.
Figure 6. Elastic recruitment is regulated by local inhibitory neurons.
(A) Absolute ictal recruitment durations vary across seizures. Box plots displaying population recruitment durations analyzed for each condition. Left: 4-AP under isoflurane (7 exp., 71 seizures). Middle: 4-AP during wakefulness (5 exp., 26 seizures). Right: Ptx during wakefulness (3 exp., 19 seizures). (B) Ictal recruitment durations vary within individual exp.. Box plots of 15 exp. (7 anesthesia, 8 wakefulness): Boxes represent 25%ile to 75%ile of cellular recruitment, bands inside boxes display median cell recruitment time. (C) Schematic depiction of exp. involving two Ptx injections during wakefulness: LFP and Ptx pipettes are located within the seizure initiation site. After LFP pipette insertion and baseline imaging, the Ptx (10mM) pipette is inserted. The 1st Ptx injection (‘Ptx-1’, blue) is volume controlled, as usual (500 nl, total amount delivered = 5 nmol]), and followed by imaging seizure spread, as described, for ~60min. Then, a 2nd Ptx injection (‘Ptx-2’, red) is performed (pressure controlled, 10 psi, 10min). (D) Merged avg images of ictal FOV post Ptx-1 (blue) and post Ptx-2 (red). Note the complete overlap of the avg images (magenta), that is, the imaged focal plane remains stable beyond Ptx-2. (E) Representative experiment. Left: Post Ptx-1, imaging (black) and LFP recordings (gray) of spreading seizures for >1hr (note: no calcium response to IIS in the LFP). Right: ~20 min post Ptx-2. (F) Magnification of inset in E. Note that post Ptx-2, every IIS in the LFP coincides with a population calcium response within the FOV in the propagation area. (G) Same exp., post Ptx-1. Left: Superimposed 2-sec windows of population calcium activity (top, individual traces in black, mean in blue) centered around 678 IIS recorded by LFP at the distant injection site (bottom, individual events in gray, mean in blue). Right: Superimposed 10-sec windows of 6 seizures recorded by imaging (top, individual seizures in black, mean in blue) and LFP (bottom, individual seizures in gray, mean in blue). Note the delay of several sec between electrographic seizure onset and optical invasion. (H) Same exp., post Ptx-2. Left: Superimposed 2-sec windows of population calcium activity (top, individual traces in black, mean in red) centered around 562 IIS (bottom, individual events in gray, mean in red). Right: Superimposed 10-sec windows of 3 seizures recorded by imaging (top, individual seizures in black, mean in red) and LFP (bottom, individual seizures in gray, mean in red). Note the clear population calcium response to IIS in the LFP and the immediate penetration of the imaged FOV upon electrographic seizure onset. (I) Quantification of optical invasion per IIS (n=4 exp., 1327 IIS post Ptx-1 [n.d. = none detectable], 893 IIS post Ptx-2 [mean invasion rate = 100%], Mann Whitney test: p = 0). (J) Superposition of all optical invasions during electrographic seizures (n=4 exp.; post Ptx-1: 21 seizures, individual events in light blue, mean in dark blue; post Ptx-2: 17 seizures, individual events in light red, mean in dark red) centered around the 50% recruitment frame of the population. Cell number in % for comparability across exp.. Note how the slow, s-curved population recruitment curve upon Ptx-1 changes into a near step-like function post Ptx-2. (K) Quantitative comparison of absolute population recruitment duration and duration standard deviation (std) of the Ptx-1 (blue) versus Ptx-2 (red) condition (n=4 exp., Ptx-1/Ptx-2: 21/17 seizures): mean recruitment duration (5.696 ± 1.915 vs. 0.375 ± 0.052 sec, Mann Whitney test: p = 0.0286), mean recruitment duration std (1.69 ± 0.625 vs. 0.068 ± 0.024 sec, p = 0.0286). (L) Quantitative comparison of absolute time delay and delay std of optical invasion after electrographic seizure onset of the Ptx-1 (blue) versus Ptx-2 (red) condition (n=4 exp., Ptx-1/Ptx-2: 21/17 seizures): mean delay (5.7 ± 0.538 vs. 0.116 ± 0.038 sec, Mann Whitney test: p = 0.002), mean delay std (1.78 ± 0.62 vs. 0.055 ± 0.012 sec, p = 0.0286).
Seizure progression elasticity is determined by local inhibition
To gain mechanistic insight into this phenomenon in vivo, we drew clues from previous work in acute brain slices, which provided evidence that interneuron activity governs the speed of ictal progression (Chagnac-Amitai and Connors, 1989; Trevelyan et al., 2007). To this end, we added a second part to the experiments involving local Ptx injection in awake mice. First, we injected Ptx (‘Ptx-1’) and carried out imaging and LFP recordings as described. Then, after repeated seizure spread into our field of view under the usual condition, we pressure injected Ptx (‘Ptx-2’) analogously to a typical OGB-1 injection (picrospritzer, ~10 psi for 10 min) (Ayzenshtat et al., 2016; Miller et al., 2014), at the same location where the first injection took place (Figure 6C). Post Ptx-2 injection, we were still able to image exactly the same FOV in the propagation area that was used post Ptx-1, without any distortion of the imaged plane (Figure 6D). When we resumed imaging and LFP recordings post Ptx-2, we found profoundly different local population dynamics in response to IIS and during seizures (Figure 6E). Prior to Ptx-2, IIS did not coincide with optical population responses in the FOV, and optical seizure break-in happened slowly (Figure 5C, 6 A right, 6 E left and 6 G). Post Ptx-2, we started to see reliable calcium responses to IIS and rapid seizure invasion (Figure 6E right, Figure 6F and H), suggesting that Ptx had diffused to the imaged territory. In fact, prior to complete drug diffusion across our field of view, it was possible to identify a demarcation line between tissue that had already been affected by the drug, and tissue where inhibition was still intact (Figure S4 D). While cells being located proximally to said demarcation line rapidly responded to IIS, brief and prolonged ictal events, distally located cells were recruited only to prolonged seizures, in a delayed fashion and at much lower speed (Figure S4 D). Importantly, the trajectory of seizure propagation across the FOV remained similar to the condition prior to Ptx-2 (Compare Figure S4 A bottom and S4 D). To quantify differences in interictal and ictal network recruitment dynamics related to Ptx-1 or Ptx-2 across animals, we compared population calcium responses to electrographic IIS (recorded at the initiation site), the time delay between electrographic seizure onset and optical seizure break-in, and the local population recruitment. Indeed, post Ptx-1, IIS were never followed by a change in local population calcium, while after Ptx-2, IIS consistently evoked a response (Figure 6I, calcium response rate per IIS [Ptx-1 vs. Ptx-2]: n = 4 exp.: 1327 vs. 893 IIS, 0 vs. 100% invasion rate, p = 0). With respect to spreading seizures, the imaged population was recruited slowly and with great temporal variability post Ptx-1, while both ictal recruitment duration and duration std decreased strongly post Ptx-2 (Figure 6J, and 6K [Ptx-1 vs. Ptx-2]: n = 4 exp.: mean recruitment duration 5.696 ± 1.915 vs. 0.375 ± 0.052 sec, p = 0.0286; mean recruitment duration std 1.69 ± 0.625 vs. 0.068 ± 0.024 sec, p = 0.0286). Similar results were obtained for the time delay between the electrographic seizure onset and optical seizure break-in (Figure 6L [Ptx-1 vs. Ptx-2]: n = 4 exp.: mean delay 5.7 ± 0.538 vs. 0.116 ± 0.038 sec, p = 0.002; mean delay std (1.78 ± 0.62 vs. 0.055 ± 0.012 sec, p = 0.0286). In conjunction with our cellular recruitment analyses, these experiments revealed that elasticity and speed of micro-scale seizure progression critically depends on the activity of local inhibitory interneurons.
Discussion
Stereotypical propagation of epileptic seizures in cortical microcircuits
Partly due to a lack of studies with appropriate temporal and spatial resolution, an ongoing debate has remained as to whether or not the recruitment of microscale networks to ictal activity happens in a stereotypical or random fashion. To address this question, we combined LFP recordings with fast resonant two-photon calcium imaging in mouse models of locally induced, acute seizures. Our findings hold true across anesthesia and wakefulness, two different seizure models (4-AP or Ptx), and cortical areas. Most experiments in this study involved imaging of somatosensory cortex (LII/III) after local 4-AP or Ptx injection at 1.5–3 mm posterior to the FOV. However, we also performed experiments where we imaged in visual cortex with the injection site being located anterior, with consistent results (Figure S5). This suggests that, at the least in the setting of acute seizures, our results i) reflect basic patterns of ictal spread, ii) are independent of the experimental model of focal onset seizures, and iii) apply across cortical areas.
Our experiments support previous work on the existence of ictal network stereotypy (Schevon et al., 2012; Truccolo et al., 2011), yet show that local network reliability is not exact. This is consistent with previous studies that show that, under normal conditions, cortical microcircuits generate repeated patterns of multicellular activity that are however never exact (Cossart et al., 2003; Ikegaya et al., 2004; Miller et al., 2014) In addition, by use of multilayer imaging we show that cellular recruitment reliability to spreading seizures is not restricted to LII/III, but holds true across cortical depth. Our results do not support reports of fully non-repeated ictal recruitment patterns (Bower et al., 2012), and further suggest that the observation of unpredictable spatiotemporal recruitment of local cell assemblies to seemingly stereotypical interictal spikes in the LFP (Feldt Muldoon et al., 2013; Muldoon et al., 2015) may not apply to the local recruitment dynamics during cortical seizure spread. In line with previous cellular resolution imaging studies in slices during interictal and ictal conditions (Badea et al., 2001; Feldt Muldoon et al., 2013; Trevelyan et al., 2006), and reports employing wide field imaging (Ma et al., 2013; Rossi, 2016), we show that ictal recruitment of local networks occurs in a spatially ordered manner.
Interlaminar stereotypical propagation of epileptic seizures
There is evidence that deep cortical layers are crucially involved in focal ictogenesis, where they precede the recruitment of superficial neurons to ictal activity (Connors, 1984; Polack et al., 2007; Rheims et al., 2008; Telfeian and Connors, 1998; Trevelyan et al., 2006), but see also(Tsau et al., 1999). However, the layer-specific neural dynamics during lateral seizure spread have hardly been studied (Adams et al., 2015; Borbely et al., 2006; Telfeian and Connors, 1998; Tsau et al., 1999), and exclusively in vitro. Our results show an initial recruitment of superficial layers during lateral spread of ictal activity, which seems in contrast to a previous study in brain slices where deep layers were observed to lead lateral seizure spread, when inhibition was moderately reduced (Telfeian and Connors, 1998). Under such conditions, seizure propagation was dependent on intact tissue bridges within LIV to LVI and was most effectively blocked when LVb was inactivated by local GABA administration. However, the same study showed that under high doses of Ptx, lateral seizure propagation was possible in each layer separately. Considering the experimental restrictions of both our study and Telfeian and Connor’s report, several factors could account for the seemingly discrepant results. First, there is the inherently impaired neural connectivity in acute brain slices, which could have influenced the previously reported findings. Even though prism insertion in our experiments is expected to have affected cortical connectivity in the vicinity of the imaged field of view as well, it did so to a lesser extent, leaving most of the anatomical and functional cortical and subcortical connections intact. Secondly, bath application of disinhibitory drugs to brain slices leads to a global impairment of inhibition, which is unlikely to take place similarly in the intact brain. While our data point away from LV and favor LII/III as the lead layer during lateral ictal spread, it remains possible that this spread could in fact be led by layer IV. This layer was consistently nearly devoid of labeled cells in our study (this has been observed in other studies as well, and cannot only be explained by injection depth (Andermann et al., 2013; Chia and Levene, 2009a)). In this case, LIV neurons could suppress LV and activate superficial layers, which subsequently signal to LV (Thomson and Bannister, 2003), a scenario convergent with known connectivity motifs in mouse somatosensory cortex (Pluta et al., 2015). Our findings bear potential implications for neurosurgical intervention in epilepsy, as they indicate - e.g. regarding sub-pial resections - that the disconnection of LII/III alone could be sufficient to stop lateral seizure spread.
Clinical relevance
This study concentrated on basic aspects of seizure spread into cortical areas that surround an acutely established epileptic focus. We capitalized on aspects that are shared between our approach and medical conditions such as brain trauma, which often present with acute seizures without the process of epileptogenesis (Beleza, 2012). Our results also carry potential implications for chronic epilepsies with focal onset seizures. There, territories outside the epileptic focus remain functionally intact, and thus resemble the local networks imaged in this study. Instead of focusing on a specific disease pathway we rather considered seizures as a phenomenon shared by many neurological disorders and even the healthy brain (Jirsa et al., 2014). Further, the approach of local injection of 4-AP or Ptx represents widely established models of partial onset seizures eliciting electrographic phenomena and behavioral symptoms that resemble those in naturally occurring epilepsy (Avoli et al., 2002; Szente and Pongracz, 1979), and have contributed successfully to therapeutic research (Gajda et al., 2005; Rothman, 2009). The total amount of injected 4-AP or Ptx was small (7.5 nmol or 5 nmol, respectively). The fact that we did not see enhanced firing in the imaged region prior to seizure break-in, neither post 4-AP nor Ptx (Ptx-1), and that particularly after local Ptx injection no change in local calcium activity was observed in response to IIS in the LFP, over the entire experiment, underscores that we imaged within the propagation area, and makes it unlikely that the imaged area was directly exposed to 4-AP or Ptx. Only after prolonged pressure injection of Ptx (Ptx-2), did we see a change in local calcium dynamics towards typical network activity manifestations induced by Ptx, which suggested that the drug had finally diffused to the imaged FOV.
We did not image the dynamics of precisely defined neural sub-populations during seizures. There is evidence, including the data presented here, that the local interaction of excitatory and inhibitory circuits is crucial for successful or failed ictal progression (Cammarota et al., 2013; Hunt et al., 2013; Khoshkhoo et al., 2017; Sessolo et al., 2015; Trevelyan et al., 2006; Ziburkus et al., 2006). While several recent studies have shown promising results with respect to cell sub-population based therapeutic seizure intervention (Krook-Magnuson et al., 2013; Ledri et al., 2014), others have raised concerns about the potential seizure promoting effects of such therapies (Avoli and de Curtis, 2011; Fujiwara-Tsukamoto et al., 2010; Gnatkovsky et al., 2008; Grasse et al., 2013; Sessolo et al., 2015). This controversy highlights that our knowledge of the basic dynamics of densely packed local ictal networks and the neural sub-types therein has remained unsatisfying. Future studies applying fast high density recordings of neural networks and sub-populations during seizures will be important in substantiating our understanding of epileptic networks and identifying novel therapeutic targets for more efficient seizure control.
Elastic cortical seizure propagation and its potential relevance for cortical function
Finally, despite a repeated relative spatiotemporal structure of seizure progression, we found variable absolute temporal recruitment of local cell populations within and across experiments. This “elastic” progression, which could stretch in time over seconds, could be interpreted in light of Hebb’s “phase sequences” (Hebb, 1949), or sequential activation of local cell assemblies, and other intrinsic network dynamics (Harris, 2005) that shape temporal activity patterns of neural cell populations (Carrillo-Reid et al., 2015; Cossart et al., 2003; Harris, 2005; Ikegaya et al., 2004; Luczak et al., 2013; MacLean et al., 2005; Scholvinck et al., 2015; Skaggs et al., 1996). Based on this model, an expanding seizure could constitute the extreme version of a phase sequence whose temporal properties are governed by local internal dynamics such as the level of sustained synaptic input or efficacy of inhibition, and global internal dynamics like state of arousal or global network synchrony. Testable factors likely affecting these dynamics with respect to seizures, could for example involve the distance from the seizure initiation site, age or gender of the studied subject, different oscillatory brain states (Ewell et al., 2015), or differentially evoked firing patterns of distinct interneuronal sub-populations (Adesnik et al., 2012). Indeed, there is evidence, for example, that velocity of ictal expansion depends on the activity of inhibitory interneurons (Prince and Wilder, 1967; Trevelyan et al., 2007). We substantiate this evidence in vivo by showing that local compromising of GABAergic signaling results in the abolishment of ictal micro-progression elasticity and a speed up of local seizure spread. However, the preexistent anatomical framework of specifically interconnected neurons restrains the possibilities of spatial seizure progression within the network.
Altogether, our finding of substantial temporal variability in combination with considerable relative spatiotemporal reliability of dense local networks during successive seizures suggests a network model of epilepsy resembling an elastic meshwork wherein ictal progression may vary in time but cannot betray preexistent neural connectivity. Our results also carry potential implications for physiological cortical processing and are in good agreement with previous studies investigating sequential ensemble activation (Ikegaya et al., 2004; Luczak et al., 2013; MacLean et al., 2005; Malvache et al., 2016; Scholvinck et al., 2015; Skaggs et al., 1996). Far from clock-driven machines, such as digital computers, it is fascinating to speculate how cortical circuits could function by activating specific dynamical trajectories that could be engaged elastically at different time scales. This flexible temporal environment could enable sensory stimuli to be integrated even if they have temporal variability, make possible for different areas of the cortex to interact in a flexible manner, and perhaps also influence an individual’s decision making depending on the differential temporal convergence of sensory information.
Experimental Procedures
Further details and an outline of resources used in this work can be found in Supplemental Experimental Procedures. All experiments were conducted with care and accordance with the Columbia University institutional animal care guidelines. Experiments were carried out on C57BL/6 adult mice at postnatal age of 1–3 months. LFP measurements and concomitant in vivo two photon calcium imaging were performed either 1hr after cortical Oregon Bapta Green-1 AM injection, 4–5 weeks after lentiviral transfer of GCaMP6s, or in transgenic Thy1-GCaMP6f animals. For experiments involving multi-layer imaging, a small glass microprism was implanted into the cortex. A part of the craniotomy remained uncovered in front of the prism face to allow access for the LFP and 4-AP micropipettes. Seizure induction was achieved through local injection of small amounts of either 4-AP or Ptx into layer V. The distance between the seizure initiation site and the imaged area ranged from 1.5 to 3 mm. Cell regions of interest (ROIs) were identified in a semi-automated fashion followed by manual confirmation. In order to identify the ictal recruitment time-point of individual cells, we used the first discrete derivative (slope) of the ΔF/F traces following electrographic seizure onset. For assessment of individual cell recruitment reliability, a time bin or relative recruitment score (rr-score) classification was used and compared to a shuffled surrogate. All data were analyzed using custom written code in MATLAB. Error bars on bar plots and shaded areas in graph plots indicate s.e.m.. Reliability indices of cells or tiles were compared using one-way ANOVA. Spatio-temporal clustering was assessed by bivariate ANOVA analysis of mean distance differences. In multilayer imaging experiments, spatial tile onset order distributions were compared to an even distribution (16 proximal/16 distal, 16 superficial/16 deep) with a 2X2 chi-square test. With respect to optical seizure invasion rate, cellular recruitment durations, duration std, LFP to calcium delays, and delay std, 2 experimental groups (Ptx-1 vs. Ptx-2, Fig. 6I, K, L) were compared across experiments by Mann Whitney test.
Supplementary Material
OGB-1 calcium imaging of full ictal event (4-AP) within propagation area, left somatosensory cortex, layer II/III (imaging depth ~150μm beneath pial surface). The incoming wave corresponds to the electrographic seizure onset. Movie is played at 3x acquisition speed.
GCaMP6 calcium imaging of full ictal event (4-AP) within propagation area, left somatosensory cortex, layer II/III (imaging depth ~150μm beneath pial surface). The incoming wave corresponds to the electrographic seizure onset. Movie is played at 3x acquisition speed.
GCaMP6 multilayer calcium imaging of ongoing spontaneous population activity prior to 4-AP injection. Left somatosensory cortex, layer II/III/V. Movie is played at 2x acquisition speed.
Same field of view as in movie S3. GCaMP6 multilayer calcium imaging of propagating seizure after 4-AP injection. Movie is played at 2x acquisition speed.
Another example of a propagating seizure (4-AP) captured by multilayer calcium imaging. Left somatosensory cortex, layer II/III/V. Movie is played at 3x acquisition speed.
GCaMP6 calcium imaging of full ictal event (4-AP) within propagation area, left somatosensory cortex, layer II/III (imaging depth ~150μm) during wakefulness. Optical break-in of ictal activity happens with seconds of delay after electrographic seizure onset (see Figure 5B). Movie is played at 3x acquisition speed.
Supplemental Experimental Procedures and Figures S1–S5
Highlights.
Seizures spread reliably through cortical microcircuits, within and across layers.
Seizures recruit supra-granular layers ahead of deep layers.
Despite relative reliability, recruitment is variable in absolute time (‘Elasticity’).
Elasticity of ictal progression is shaped by local GABAergic interneurons.
Acknowledgments
We thank Dr. Yeonsook Shin and Alexa Semonche for viral injections and to members of the Yuste Lab for comments. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, grant WE 5517/1-1), NEI (DP1EY024503, R01EY011787), NIMH (R01MH101218, R01MH100561) and DARPA SIMPLEX N66001-15-C-4032. This material is based upon work supported by, or in part by, the U. S. Army Research Laboratory and the U. S. Army Research Office under contract number W911NF-12-1-0594 (MURI). The authors have no competing financial interests to declare.
Footnotes
Conflict of Interest: The authors declare no competing financial interests
Author contributions: M.W. and R.Y. conceived of the project. M.W. performed all experiments and wrote the paper. M.W. and J.P.H. analyzed the data. All authors planned experiments, discussed results, and edited the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams C, Adams NE, Traub RD, Whittington MA. Electrographic waveform structure predicts laminar focus location in a model of temporal lobe seizures in vitro. PLoS One. 2015;10:e0121676. doi: 10.1371/journal.pone.0121676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, Gilfoy NB, Goldey GJ, Sachdev RN, Wolfel M, McCormick DA, Reid RC, Levene MJ. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron. 2013;80:900–913. doi: 10.1016/j.neuron.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, D’Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Progress in neurobiology. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayzenshtat I, Karnani MM, Jackson J, Yuste R. Cortical Control of Spatial Resolution by VIP+ Interneurons. J Neurosci. 2016;36:11498–11509. doi: 10.1523/JNEUROSCI.1920-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea T, Goldberg J, Mao B, Yuste R. Calcium imaging of epileptiform events with single-cell resolution. J Neurobiol. 2001:215–227. doi: 10.1002/neu.1052. [DOI] [PubMed] [Google Scholar]
- Baird-Daniel E, Daniel AG, Wenzel M, Li D, Liou JY, Laffont P, Zhao M, Yuste R, Ma H, Schwartz TH. Glial Calcium Waves are Triggered by Seizure Activity and Not Essential for Initiating Ictal Onset or Neurovascular Coupling. Cerebral cortex. 2017:1–13. doi: 10.1093/cercor/bhx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Loscher W. What new modeling approaches will help us identify promising drug treatments? Advances in experimental medicine and biology. 2014;813:283–294. doi: 10.1007/978-94-017-8914-1_23. [DOI] [PubMed] [Google Scholar]
- Beleza P. Acute symptomatic seizures: a clinically oriented review. Neurologist. 2012;18:109–119. doi: 10.1097/NRL.0b013e318251e6c3. [DOI] [PubMed] [Google Scholar]
- Borbely S, Halasy K, Somogyvari Z, Detari L, Vilagi I. Laminar analysis of initiation and spread of epileptiform discharges in three in vitro models. Brain Res Bull. 2006;69:161–167. doi: 10.1016/j.brainresbull.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Bower MR, Stead M, Meyer FB, Marsh WR, Worrell GA. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia. 2012;53:807–816. doi: 10.1111/j.1528-1167.2012.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Losi G, Chiavegato A, Zonta M, Carmignoto G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. The Journal of physiology. 2013;591:807–822. doi: 10.1113/jphysiol.2012.238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L, Miller JEK, Hamm JP, Jackson J, Yuste R. Endogenous sequential cortical activity evoked by visual stimuli. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:8813–8828. doi: 10.1523/JNEUROSCI.5214-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol. 1989;61:747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia TH, Levene MJ. In vivo imaging of deep cortical layers using a microprism. Journal of visualized experiments: JoVE. 2009a:e1509. doi: 10.3791/1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia TH, Levene MJ. Microprisms for in vivo multilayer cortical imaging. J Neurophysiol. 2009b;102:1310–1314. doi: 10.1152/jn.91208.2008. [DOI] [PubMed] [Google Scholar]
- Connors BW. Initiation of synchronized neuronal bursting in neocortex. Nature. 1984;310:685–687. doi: 10.1038/310685a0. [DOI] [PubMed] [Google Scholar]
- Cossart R, Aronov D, Yuste R. Attractor dynamics of network UP states in neocortex. Nature. 2003;423:283–289. doi: 10.1038/nature01614. [DOI] [PubMed] [Google Scholar]
- Cymerblit-Sabba A, Schiller Y. Development of hypersynchrony in the cortical network during chemoconvulsant-induced epileptic seizures in vivo. J Neurophysiol. 2012;107:1718–1730. doi: 10.1152/jn.00327.2011. [DOI] [PubMed] [Google Scholar]
- Dana H, Chen TW, Hu A, Shields BC, Guo C, Looger LL, Kim DS, Svoboda K. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One. 2014;9:e108697. doi: 10.1371/journal.pone.0108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewell LA, Liang L, Armstrong C, Soltesz I, Leutgeb S, Leutgeb JK. Brain State Is a Major Factor in Preseizure Hippocampal Network Activity and Influences Success of Seizure Intervention. J Neurosci. 2015;35:15635–15648. doi: 10.1523/JNEUROSCI.5112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt Muldoon S, Soltesz I, Cossart R. Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. PNAS. 2013;110:3567–3572. doi: 10.1073/pnas.1216958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Imanishi M, Ninomiya T, Tsukada M, Yanagawa Y, Fukai T, Takada M. Prototypic seizure activity driven by mature hippocampal fast-spiking interneurons. J Neurosci. 2010;30:13679–13689. doi: 10.1523/JNEUROSCI.1523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda Z, Szupera Z, Blazso G, Szente M. Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia. 2005;46:1581–1591. doi: 10.1111/j.1528-1167.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Annals of neurology. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- Grasse DW, Karunakaran S, Moxon KA. Neuronal synchrony and the transition to spontaneous seizures. Experimental neurology. 2013;248:72–84. doi: 10.1016/j.expneurol.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat Rev Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nature neuroscience. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Mechanisms of propagation: extracellular studies. In: Jasper HH, Ward AA, Pope A, editors. Basic mechanisms of the epilepsies. New York: Little, Brown; 1969. [Google Scholar]
- Jirsa VK, Stacey WC, Quilichini PP, Ivanov AI, Bernard C. On the nature of seizure dynamics. Brain. 2014;137:2210–2230. doi: 10.1093/brain/awu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Truccolo W, Gale JT, Eskandar E, Thesen T, Carlson C, Devinsky O, Kuzniecky R, Doyle WK, Madsen JR, et al. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain. 2010;133:1668–1681. doi: 10.1093/brain/awq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshkhoo S, Vogt D, Sohal VS. Dynamic, Cell-Type-Specific Roles for GABAergic Interneurons in a Mouse Model of Optogenetically Inducible Seizures. Neuron. 2017;93:291–298. doi: 10.1016/j.neuron.2016.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. A functional comparison of the antagonists bicuculline and picrotoxin at recombinant GABAA receptors. Neuropharmacology. 1996;35:1289–1298. doi: 10.1016/s0028-3908(96)00089-5. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature communications. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nature neuroscience. 2015;18:331–338. doi: 10.1038/nn.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledri M, Madsen MG, Nikitidou L, Kirik D, Kokaia M. Global optogenetic activation of inhibitory interneurons during epileptiform activity. J Neurosci. 2014;34:3364–3377. doi: 10.1523/JNEUROSCI.2734-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis KP, Wang Z, Mail M, Zhao GQ, Berdichevsky Y, Bacskai B, Staley KJ. Evolution of Network Synchronization during Early Epileptogenesis Parallels Synaptic Circuit Alterations. J Neurosci. 2015;35:9920–9934. doi: 10.1523/JNEUROSCI.4007-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Harris KD. Gating of sensory input by spontaneous cortical activity. J Neurosci. 2013;33:1684–1695. doi: 10.1523/JNEUROSCI.2928-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhao M, Schwartz TH. Dynamic neurovascular coupling and uncoupling during ictal onset, propagation, and termination revealed by simultaneous in vivo optical imaging of neural activity and local blood volume. Cerebral cortex. 2013;23:885–899. doi: 10.1093/cercor/bhs079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Malvache A, Reichinnek S, Villette V, Haimerl C, Cossart R. Awake hippocampal reactivations project onto orthogonal neuronal assemblies. Science. 2016;353:1280–1283. doi: 10.1126/science.aaf3319. [DOI] [PubMed] [Google Scholar]
- Martinet LE, Ahmed OJ, Lepage KQ, Cash SS, Kramer MA. Slow Spatial Recruitment of Neocortex during Secondarily Generalized Seizures and Its Relation to Surgical Outcome. J Neurosci. 2015;35:9477–9490. doi: 10.1523/JNEUROSCI.0049-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Ayzenshtat I, Carrillo-Reid L, Yuste R. Visual stimuli recruit intrinsically generated cortical ensembles. PNAS. 2014;111:E4053–4061. doi: 10.1073/pnas.1406077111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Villagran A, Tapia R. Preferential stimulation of glutamate release by 4-aminopyridine in rat striatum in vivo. Neurochem Int. 1996;28:35–40. doi: 10.1016/0197-0186(95)00064-f. [DOI] [PubMed] [Google Scholar]
- Muldoon SF, Villette V, Tressard T, Malvache A, Reichinnek S, Bartolomei F, Cossart R. GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain. 2015;138:2875–90. doi: 10.1093/brain/awv227. [DOI] [PubMed] [Google Scholar]
- Neubauer FB, Sederberg A, MacLean JN. Local changes in neocortical circuit dynamics coincide with the spread of seizures to thalamus in a model of epilepsy. Frontiers in neural circuits. 2014;8:101. doi: 10.3389/fncir.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta S, Naka A, Veit J, Telian G, Yao L, Hakim R, Taylor D, Adesnik H. A direct translaminar inhibitory circuit tunes cortical output. Nature neuroscience. 2015;18:1631–1640. doi: 10.1038/nn.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Archives of neurology. 1967;16:194–202. doi: 10.1001/archneur.1967.00470200082007. [DOI] [PubMed] [Google Scholar]
- Rheims S, Represa A, Ben-Ari Y, Zilberter Y. Layer-specific generation and propagation of seizures in slices of developing neocortex: role of excitatory GABAergic synapses. J Neurophysiol. 2008;100:620–628. doi: 10.1152/jn.90403.2008. [DOI] [PubMed] [Google Scholar]
- Rossi LFW, RC, Kullman D, Carandini M. Cortical seizure propagation respects functional connectivity underlying sensory processing. bioRxiv 2016 [Google Scholar]
- Rothman SM. The therapeutic potential of focal cooling for neocortical epilepsy. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:251–257. doi: 10.1016/j.nurt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G, Jr, Goodman RR, Yuste R, Emerson RG, Trevelyan AJ. Evidence of an inhibitory restraint of seizure activity in humans. Nature communications. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Saleem AB, Benucci A, Harris KD, Carandini M. Cortical state determines global variability and correlations in visual cortex. J Neurosci. 2015;35:170–178. doi: 10.1523/JNEUROSCI.4994-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessolo M, Marcon I, Bovetti S, Losi G, Cammarota M, Ratto GM, Fellin T, Carmignoto G. Parvalbumin-Positive Inhibitory Interneurons Oppose Propagation But Favor Generation of Focal Epileptiform Activity. J Neurosci. 2015;35:9544–9557. doi: 10.1523/JNEUROSCI.5117-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. PNAS. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo GG, Schneider CJ, Soltesz I. Resolution revolution: epilepsy dynamics at the microscale. Current opinion in neurobiology. 2015;31:239–243. doi: 10.1016/j.conb.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szente M, Pongracz F. Aminopyridine-induced seizure activity. Electroencephalography and clinical neurophysiology. 1979;46:605–608. doi: 10.1016/0013-4694(79)90014-2. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Goldberg J, Yuste R. Calcium oscillations in neocortical astrocytes under epileptiform conditions. Journal of neurobiology. 2002;50:45–55. doi: 10.1002/neu.10019. [DOI] [PubMed] [Google Scholar]
- Telfeian AE, Connors BW. Layer-specific pathways for the horizontal propagation of epileptiform discharges in neocortex. Epilepsia. 1998;39:700–708. doi: 10.1111/j.1528-1157.1998.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cerebral cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Watson BO, Yuste R. Modular Propagation of Epileptiform Activity: Evidence for an Inhibitory Veto in Neocortex. J Neurosci. 2006:12447–12455. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Yuste R. Feedforward Inhibition Contributes to the Control of Epileptiform Propagation Speed. J Neurosci. 2007:3383–3387. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Ahmed OJ, Harrison MT, Eskandar EN, Cosgrove GR, Madsen JR, Blum AS, Potter NS, Hochberg LR, Cash SS. Neuronal ensemble synchrony during human focal seizures. J Neurosci. 2014;34:9927–9944. doi: 10.1523/JNEUROSCI.4567-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Single-neuron dynamics in human focal epilepsy. Nature neuroscience. 2011;14:635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsau Y, Guan L, Wu JY. Epileptiform activity can be initiated in various neocortical layers: an optical imaging study. J Neurophysiol. 1999:1965–1973. doi: 10.1152/jn.1999.82.4.1965. [DOI] [PubMed] [Google Scholar]
- Wong BY, Prince DA. The lateral spread of ictal discharges in neocortical brain slices. Epilepsy research. 1990;7:29–39. doi: 10.1016/0920-1211(90)90051-v. [DOI] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OGB-1 calcium imaging of full ictal event (4-AP) within propagation area, left somatosensory cortex, layer II/III (imaging depth ~150μm beneath pial surface). The incoming wave corresponds to the electrographic seizure onset. Movie is played at 3x acquisition speed.
GCaMP6 calcium imaging of full ictal event (4-AP) within propagation area, left somatosensory cortex, layer II/III (imaging depth ~150μm beneath pial surface). The incoming wave corresponds to the electrographic seizure onset. Movie is played at 3x acquisition speed.
GCaMP6 multilayer calcium imaging of ongoing spontaneous population activity prior to 4-AP injection. Left somatosensory cortex, layer II/III/V. Movie is played at 2x acquisition speed.
Same field of view as in movie S3. GCaMP6 multilayer calcium imaging of propagating seizure after 4-AP injection. Movie is played at 2x acquisition speed.
Another example of a propagating seizure (4-AP) captured by multilayer calcium imaging. Left somatosensory cortex, layer II/III/V. Movie is played at 3x acquisition speed.
GCaMP6 calcium imaging of full ictal event (4-AP) within propagation area, left somatosensory cortex, layer II/III (imaging depth ~150μm) during wakefulness. Optical break-in of ictal activity happens with seconds of delay after electrographic seizure onset (see Figure 5B). Movie is played at 3x acquisition speed.
Supplemental Experimental Procedures and Figures S1–S5