Figure 4. STAT3 inhibition decreases self-renewal in LEPRb-expressing non-CSCs.
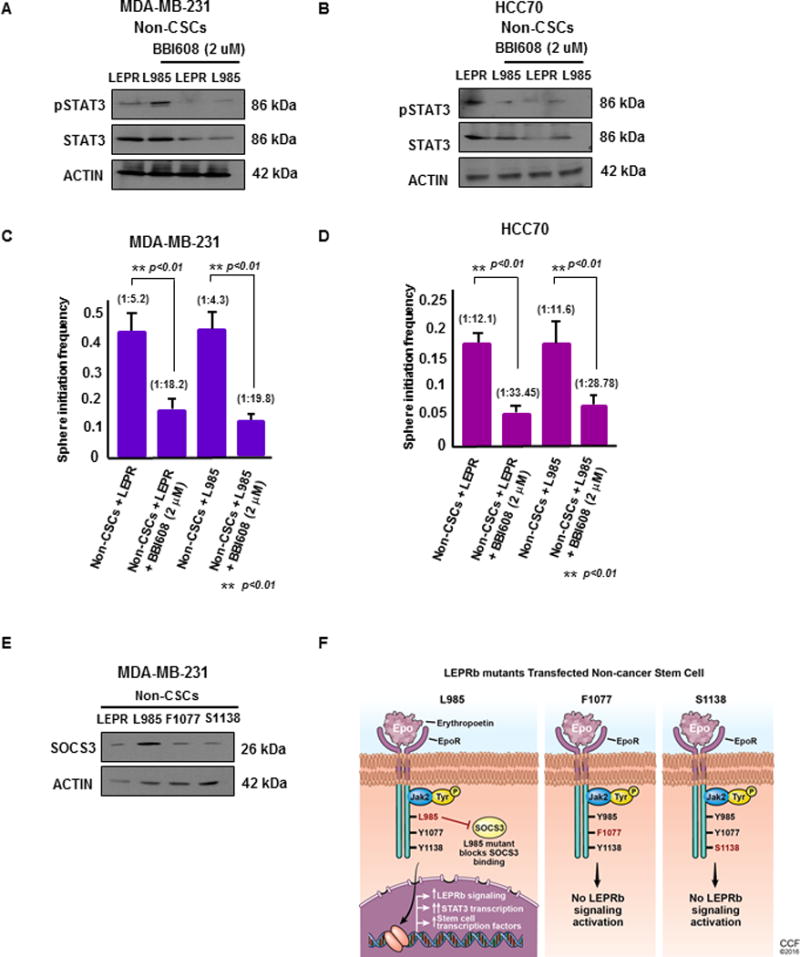
(A & B) Upon treatment with BBI608, a STAT3 pathway inhibitor, at 2 μM for 24 hours, non-CSCs (MDA-MB-231 and HCC70) transfected with LEPRb and L985 showed decreased expression of total STAT3 by immunoblotting. Actin was used as a loading control. Twenty micrograms of protein per sample was loaded into each well for the immunoblots. (C & D) Limiting dilution analyses of LEPR- and L985-transfected non-CSCs treated with BBI608 were performed. The LEPR and L985-transfected non-CSCs treated with BBI608 showed a significant decrease in stem cell frequency compared with the untreated MDA-MB-231 and HCC70 non-CSCs (p < 0.01). (E) Immunoblotting of LEPRb and LEPRb mutant (L985, F1077, S1138)-transfected non-CSCs treated with LEP (25 ng/ml) showed increased SOCS3 expression in L985-transfected non-CSCs compared with the other groups. Actin was used as a loading control. Twenty micrograms of protein per sample was loaded onto each well for the immunoblots. (F) Schematic of the transfection of the LepR L985, F1077 and S1138 mutants into non-CSCs. These tyrosine mutations were made in a chimeric protein containing the erythropoietin (Epo) receptor extracellular domain and the intracellular domain from the long form of the mouse leptin receptor containing the tyrosines. In this chimeric receptor, the activation of LepR-dependent signals occurs under the control of Epo stimulation. Upon Epo stimulation, the intracellular LepR domain maintains the same intracellular signaling program induced by native LepR. In the presence of the L985 mutation, the downstream signaling effects are still activated, due to its inability to bind to SOCS3, the inhibitor of LepR signaling. This leads to constitutively activated downstream LepR signaling, which includes activation of STAT3 and its downstream target genes. Upon introduction of F1077 and S1138, STAT5 and STAT3 transcriptional activation was blocked, leading to the inhibition of LepR signaling pathways.
