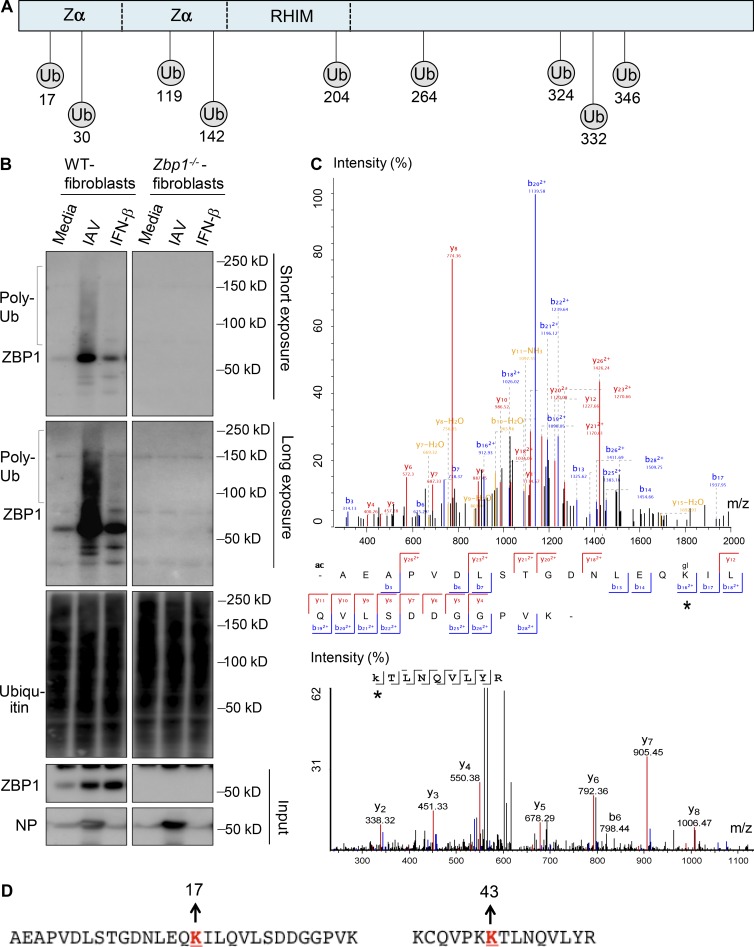
Figure 3.
IAV infection induces ZBP1 ubiquitination. (A) Schematic representation of predicted ubiquitination (Ub) sites in ZBP1. UbPred and UbiSite servers were used to predict potential ubiquitination sites in ZBP1. (B) ZBP1 was ubiquitinated after IAV infection. WT and Zbp1−/− cells were infected with IAV or treated with IFN-β (100U/ml). Whole cell lysates were harvested after 8 h of infection, to purify polyubiquitinated protein fraction by using TUBEs (tandem ubiquitin-binding entities). Purified fractions were subjected to Western blot analysis for ubiquitin and ZBP1 to detect its ubiquitination (n = 4). Poly-Ub, polyubiquitinated protein. (C) Peptide spectrum match of the ubiquitinated peptide at K17 and K43 positions. ZBP1 was immunoprecipitated from IAV infected cells and subjected to protein fragmentation. Peptides were separated by a reverse phase liquid chromatography using a Proxeon Nano-UPLC system and analyzed on a Q-Exactive HF mass spectrometer. Asterisk represents ubiquitination detected by mass spectrometry. (D) Amino acid sequence context for detected ubiquitinated ZBP1 fragments indicating K17 and K43 positions as ubiquitination sites.