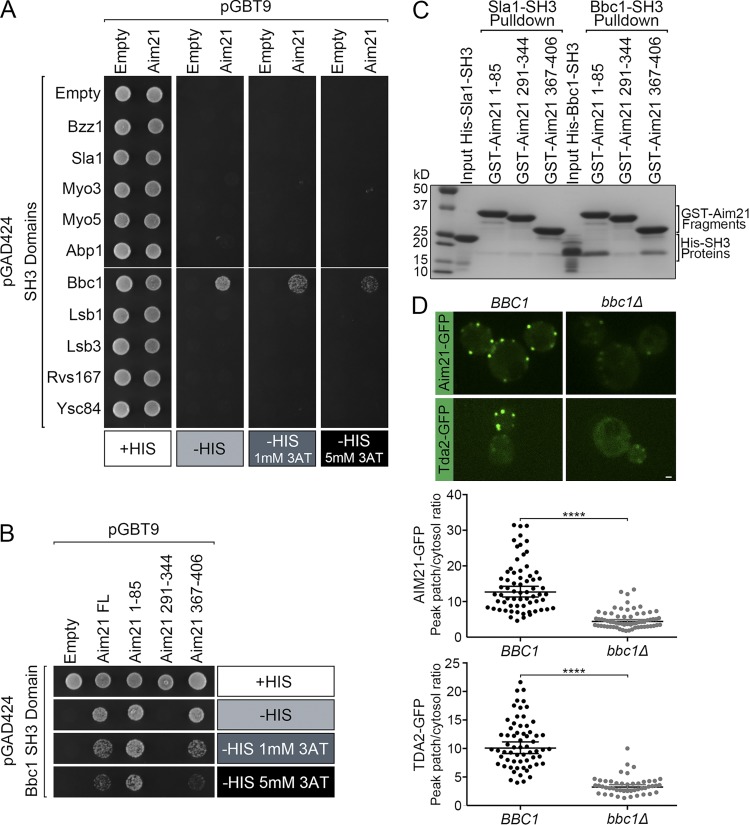
Figure 6.
Aim21 interacts physically with Bbc1, and recruitment of the Tda2–Aim21 complex to CME sites depends on Bbc1. (A) Yeast two-hybrid analysis: yeast cells were cotransformed with plasmids expressing the GAL4 DNA binding (pGBT9) and activation (pGAD424) domains fused to Aim21 and the SH3 domains from the indicated endocytic proteins, respectively. Cells were spotted onto plates containing histidine (+HIS; as control) or selective medium lacking histidine (−HIS) and containing various concentrations of 3-AT. Cell growth indicative of interaction was only detected within the Bbc1 SH3 domain. (B) Yeast two-hybrid analysis with polyproline motif–rich fragments of Aim21 (amino acids 1–85, 291–344, and 367–406) and the Bbc1 SH3 domain. Aim21 fragment 1–85 demonstrated the strongest interaction. (C) A GST pulldown assay was performed with GST fused to Aim21 fragments 1–85, 291–344, and 367–406. Each GST fusion protein was incubated with His-Sla1-SH3 and His-Bbc1-SH3. The bound proteins were analyzed by Coomassie staining demonstrating direct binding between the Bbc1 SH3 domain and Aim21. Fragment 1–85 showed the strongest binding. (D, top) Live-cell fluorescence microscopy showing recruitment of Aim21-GFP and Tda2-GFP to endocytic sites is dramatically reduced in bbc1Δ cells relative to WT cells (BBC1). (Bottom) Quantification of the Aim21-GFP and Tda2-GFP peak fluorescence intensity at endocytic patches in control (BBC1) and bbc1Δ cells. Bar, 1 µm. Error bars indicate means ± SEM. ****, P < 0.0001.