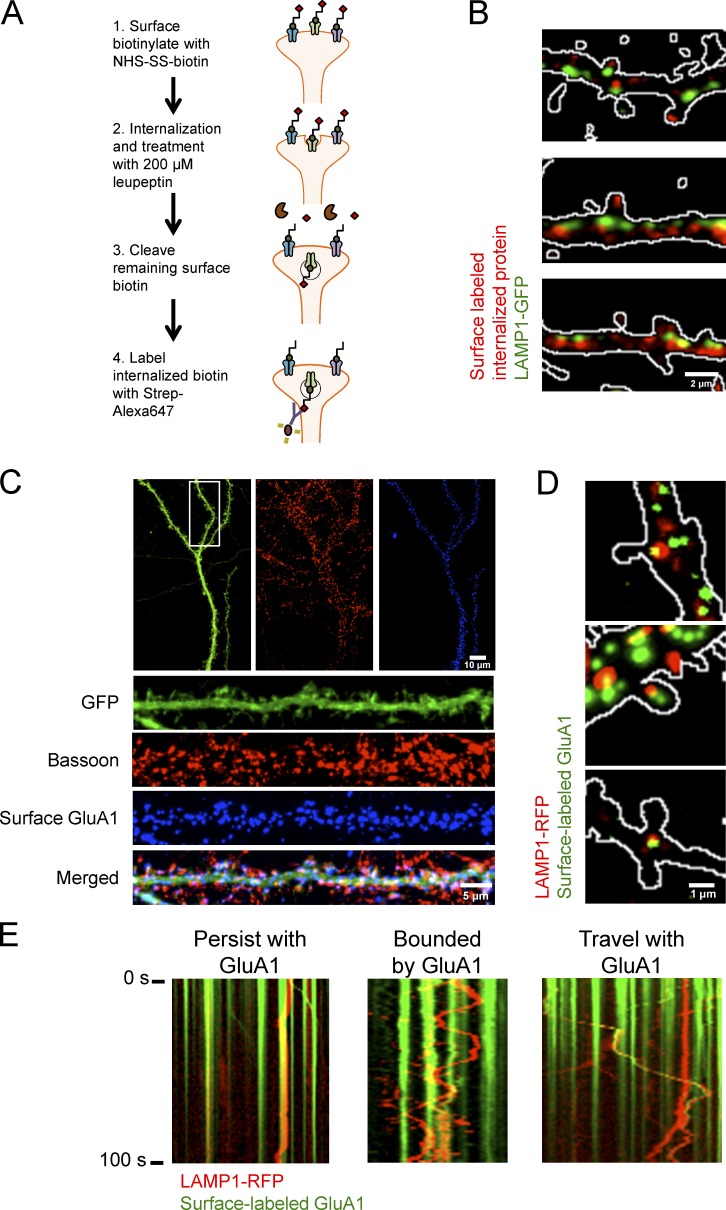
Figure 6.
Distribution and trafficking of lysosomes is highly correlated with internalized membrane proteins and synaptic AMPARs. (A) Model of bulk surface membrane internalization assay. (B) Representative images of dissociated hippocampal neurons expressing LAMP1-GFP (green) and internalized membrane proteins (red). Outlines of dendritic spines were generated by outlining the mCherry signal. (C) Representative immunofluorescent images of dissociated hippocampal neurons (16 DIV) expressing GFP and GFP-GluA1. Surface GFP-GluA1 was labeled with Alexa Fluor 647 and subsequently stained for Bassoon, a presynaptic marker. Surface GluA1 is juxtaposed to Bassoon. (D) Representative images of dissociated hippocampal cultures (DIV16) transfected with GFP, GFP-GluA1, and LAMP1-RFP. GFP-GluA1 can colocalize with a lysosome in and at the base of a dendritic spine. Cultures were treated with 100 µg/ml leupeptin and live-labeled with GFP antibody conjugated to Alexa Fluor 647. After washout, cultures were treated with 100 µM AMPA for 10 min to promote endocytosis. Outlines of dendritic spines were generated by outlining the GFP signal. Surface GFP-GluA1 labeled with anti-GFP Alexa Fluor 647 was false-colored green to show colocalization with LAMP1-RFP. (E) Live imaging of labeled surface GluA1 with LAMP1-RFP after 100 µM AMPA treatment. LAMP1-RFP labeled lysosomes persist at a location of surface-labeled AMPARs, rapidly move bidirectionally between two sites of surface-labeled AMPARs, and cotraffic with surface-labeled AMPARs likely destined for degradation.