Abstract
Stunting remains a major public health concern worldwide. Although its global prevalence is slowly decreasing, the actual number of affected children is still rising in Sub-Saharan Africa. In the Central African Republic (CAR), about one third of all children below the age of five are stunted. Stunting is correlated with many long-term consequences, including poor cognitive development and a higher rate of morbidity and mortality, making stunting a major contributor to poverty. In CAR, little is known about the factors that contribute to stunting. This study aimed at analysing, in a cross-sectional study, the main factors associated with stunting in a group of 414 children recruited between December 2011 and November 2013, aged five years or less and living in Bangui. For all children, demographic, socio-economic and anthropometric data were recorded and asymptomatic enteropathogen carriage was assessed in stool samples using classical microbiological assays. The study group had a mean age of 14.2±10 months. Fifty-eight percent (292/414) were boys, and 36 percent (148/414) exhibited stunted growth. Of the stunted children, 51% (75/148) showed a moderate delay in linear growth for their age group [height-for-age z-score (HAZ) between -2 and -3 SD] while 49% (73/148) presented a severe delay (HAZ < -3). Factors significantly associated with stunting included gender (aOR: 1.67; 95% CI: 1.07; 2.62 for boys compared to girls) and age (aOR of 3.98 (95% CI: 2.45; 6.46) for toddlers and aOR 4.42 (95% CI: 2.36; 8.28) for children compared to infants). Most importantly, we identified being overweight [weight-for-height z-score (WHZ) > 2 SD; aOR: 3.21; 95% CI: 1.50; 6.90 of overweight compared to normal weight] as also being significantly associated with stunting. This is the first study showing that even in the poorest countries of the world there is an association of stunting with being overweight.
Introduction
Globally, one out of four children (25%) under five years of age experiences developmental and growth delays (stunting). Of these stunted children, 90% live in Sub-Saharan Africa and Asia (Levels and Trends in Child Malnutrition, WHO, UNICEF, World Bank, 2012). Stunting leads to deleterious effects on the child’s short-term and long-term health, including increased susceptibility to infection and impaired brain development [1–6]. A meta-analysis of 53 767 children living in Latin America, Asia and Africa showed more than a three-fold higher mortality in stunted compared to well-nourished children [7]. In Central African Republic (CAR), where this study was carried out, the percentage of stunted children under 5 years of age is alarmingly high at 41–43% (The World Bank and Global Nutrition report, data 2010). Stunting is a complex condition that may reflect several aetiologies, such as suboptimal breastfeeding in the first months of life, a poor and unbalanced diet and/or insufficient vitamin and/or micronutrient intake thereafter. Indirect factors that may also affect healthy growth include access to healthcare, education, wealth, political stability, social support networks, urbanisation and living conditions. The influence of both direct and indirect factors was reviewed recently in the WHO Conceptual Framework on Childhood stunting and is summarized in Dewey et al, 2011 [5].
Stunting in developing countries often starts in utero and its severity increases until it reaches a plateau at about two years of age, a time period called the “1000 days” [8,9]. Notably, a recent meta-analysis of 42 studies showed that complementary feeding practices to overcome chronic undernutrition were at best able to correct for roughly a third of the encountered growth deficits [10]. This finding suggests that other factors may be implicated in stunting. Factors that have been linked to stunting include poor hygiene and sanitation and recurrent gastrointestinal infections [4,11–17].
However, little data exists on the factors associated with stunting in central Africa, mostly due to recurrent political instabilities that make data collection challenging. For the CAR, we could find only a single study on risk factors associated with stunting. This study used data collected in a demographic health survey in the year 2000 and included 12 949 children aged 0–59 months. Poor socio-economic status of the family was found as the main risk factor for stunting [18]. Given the little information we have on risk factors of stunting in the CAR and its high prevalence of stunting, there is an urgent need to further investigate the factors underlying linear growth failure in this part of the world in greater detail in order to design better prevention and intervention strategies.
Stunted children are more susceptible to infections, particularly diarrhoeal and respiratory diseases [19] as well as malaria [10,20]. Infections enhance undernutrition, thus creating a vicious cycle leading to growth defects. In different animal models, it was shown that malnourished mice experimentally infected with Cryptosporidium spp. [21], entero-aggregative Escherichia coli [22,23], Giardia lamblia [24], or a cocktail of different non-pathogenic faecal bacteria [25] showed signs of enteropathy and displayed delayed growth.
Similarly, a longitudinal study performed on 197 children aged 2 to 48 months in rural Bangladesh in 1978 and 1979 revealed an association of Shigella-mediated diarrhoea and subsequent delays in linear growth (0.055 cm less growth/percent days, P = 0.008) while no association was found with enterotoxic E. coli-mediated diarrhoea and subsequent linear growth of infected children [26]. Another longitudinal study conducted between 1989–1991 on Cryptosporidium parvum in a cohort of 185 children aged 0–3 months living in Lima (Peru) followed for two years revealed a significant negative association between linear growth and Cryptosporidum parvum infection as well as the efficiency of catch-up growth [27], while another longitudinal study conducted on 545 children followed from birth to three years of age in a rural coastal region of Kenya in 2007–2010 showed a positive association between infection with Ascaris sp., soil-transmitted helminths, Giardia and malaria and linear growth failure (decreased height when infected with Ascaris at month 24: -0.93 cm, p<0.001, with soil transmitted helminths at month 24–0.35cm, p = 0.01, with Giardia spp. at month 12–0.5 cm, p = 0.003 and with malaria at month 18: -0.63 cm, p = 0.003). However, these associations were not found in all age groups tested, indicating a complex interplay between infection and subsequent stunting [28]. Notably, because of reciprocal exacerbation of undernutrition and infection, it has been difficult to distinguish between cause and effect in these epidemiological studies. In addition, many of these epidemiological studies suffer from a lack of follow up studies to confirm and consolidate the results. The direct effect of pathogens on child stunting therefore still remains poorly understood.
Interestingly, there is growing evidence that children not suffering from diarrhoea asymptomatically carry diarrhoeal pathogens [29,30,31]. The Torcadia study, a matched case-control study performed between November 2011 and December 2013 in Bangui, the capital of Central African Republic [30], sought to determine the most important pathogens in severe childhood diarrhoea leading to hospitalization among children under five years old. A considerable number of asymptomatic pathogen carriage was observed among the group of non-diarrheal children [30]. However, this data set was not analysed for risk factors associated with stunting and whether there is a relationship between this asymptomatic enteropathogen carriage and stunting. Indeed, a recent study suggests that asymptomatic pathogen carriage may be associated with stunting [32].
In this study, we performed a secondary data analysis on all healthy children included in the Torcadia study. This cross-sectional study aimed to determine the risk factors associated with stunting in children under 5 years of age living in Bangui, the capital of CAR. The secondary objective was to assess if asymptomatic pathogen carriage is associated with stunting in seemingly healthy children.
Material and methods
Data source
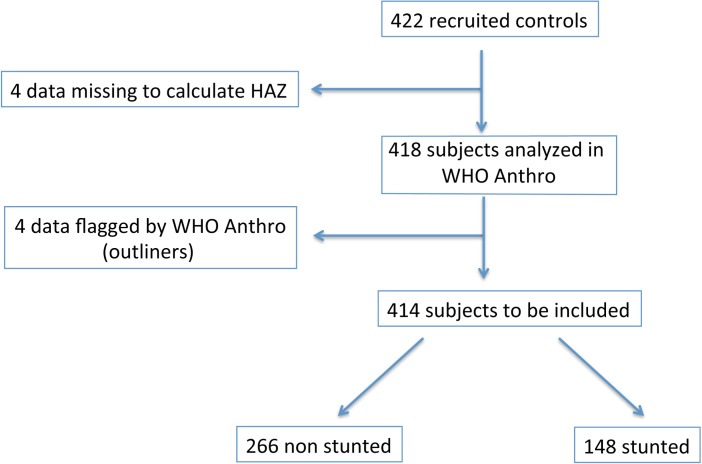
This was a cross-sectional study on all children not suffering of diarrhoea (n = 422, non hospitalized, healthy children) included in the Torcadia study, a study conducted in Bangui between November 2011 and December 2013 [30]. The inclusion criteria of these children were the following: (1) aged between 0–59 months; (2) no history of diarrhoea or antibiotics in the 7 days prior to inclusion; (3) in good general health; (4) recruited in the community; and (5) written consent by the legal representative to participate in the study. Exclusion criterion was a caregiver’s spontaneous declaration of HIV-positive status of their children. The children were studied at their homes and samples were collected and questionnaires administered by a dedicated study nurse. All healthy children with a valid height-for-age z-score (n = 414) were included in the final analysis (see flow-chart in Fig 1).
Fig 1. Flow-chart of the subjects included in the study.
Ethics statement
The initial study protocol was approved by the Comité de Recherche Clinique of the Institut Pasteur and the National Committee of the Central African Republic. The protocol included written consent for participation in the study and subsequent use of the samples and data was requested from all parents or guardians of any child participant on their behalf.
Collected variables
The collected variables include anthropometric measurements (weight, height/length, mid-upper arm circumference (MUAC), and head circumference), age, gender, family structure, different indicators of socioeconomic status (profession of parents, working situation, household size, description of living conditions, general luxury goods, mobility…), different indicators of sanitary status (drinking water, eating with fingers, access to sanitary facilities, …), and S2 Table for pathogen list).
Anthropometric variables were defined according to the WHO Growth Child standards 2006 [33,34] and calculated using the World Health Organization WHO Anthro software (version 3.2.2, January 2011). The following variables were calculated: height-for-age z-score (HAZ), Weight-for-age z-score (WAZ), BMI-for-age z-score (zBMI), Weight-for-height z-score. The following cut-offs as defined by the WHO were used: stunted: < -2 HAZ (moderately stunted: -3 ≤ HAZ < -2; severely stunted: HAZ < -3); acute malnutrition based on WHZ score: < -2 WHZ (acute malnutrition: -3 ≤ WHZ < -2; severe acute malnutrition: WHZ < -3). The definition of being overweight was based on zBMI (Normal weight: -2 SD≤ zBMI≤ 2SD, Overweight: 3≤ zBMI > 2 SD and obese zBMI > 3 SD), as this measure was shown to be a good predictor of actual body fat [35]. As to facilitate the multivariate analysis WHZ was used to indicate both, under- and overnutrition, in the logistic regression.
The microbiological analyses used included the following detection methods of pathogen detection of stool samples: Shigella spp. and Salmonella enterica, on Hektoen Enteric agar (Bio-Rad, Marnes-la-Coquette, France), Escherichia coli and other Gram-negative bacteria on bromocresol purple lactose agar and Levine's eosin-methylene blue agar (Bio-Rad, Marnes-la-Coquette, France), Yersinia enterocolitica on Cefsulodin-irgasan-novobiocin agar (Bio-Rad, Marnes-la-Coquette, France), and Vibrio cholerae on thiosulfate—citrate—bile salts—sucrose agar (Bio-Rad, Marnes-la-Coquette, France) after an initial selective enrichment with alkaline peptone water.
E. coli pathotypes were determined on a pooled sample from five putative E. coli colonies from every stool sample using a single-test multiplex PCR as previously described [36]: typical EPEC (bfpB positive), atypical EPEC (escV positive, bfp negative, stx negative), STEC (escV positive/negative, bfp negative, stx1 positive, stx2 positive, or both), ETEC (elt positive, estIa positive, estIb positive), EIEC (invE positive) and EAEC (aggR positive or astA positive with pic positive). The E. coli-specific uidA gene was used to confirm that the collected colonies were indeed E. coli. DNA from faecal samples was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Courtaboeuf, France). To improve assessment of the involvement of Shigella, a PCR assay based on amplifying the invasion plasmid antigen H (ipaH) gene contained in EIEC and Shigella spp was performed.
For the detection of group A rotaviruses, astroviruses and adenoviruses, the ProSpecT kits (Oxoid, Thermo Fisher Scientific, Basingstoke, UK) were used. To detect noroviruses of the genogroups (GG) I and II, the IDEIA Norovirus kit (Oxoid, Thermo Fisher Scientific, Basingstoke, UK). All kits were used according to the manufacturer’s instructions.
For the parasitic analyses, a subsample of the stools was concentrated by the merthiolate iodine formaldehyde concentration technique and examined for helminth eggs and protozoa cysts. Differentiation of pathogenic Entamoeba histolytica from non-pathogenic Entamoeba dispar was performed by enzyme-linked immunosorbent assay (ELISA) (Fumouze Diagnostics, France). Multiplex PCR was used for the detection of Cryptosporidium hominis and Cryptosporidium parvum on extracted DNA from feces. For a detailed description of the microbiological detection methods used, see reference [30] and [36].
Statistical analyses
The statistical analysis was performed with Stata 13. Significance level was fixed for all analyses at 0.05 and all tests performed were bilateral. Quantitative variables were expressed as mean (± Standard Deviation), or median (interquartile range); qualitative variables were expressed as percentage. The stunted vs. non-stunted groups were compared using Chi2 or Fisher Exact test for qualitative variables and the Student t Test or the Mann-Whitney U test for quantitative variables. Factors potentially associated with stunting in univariate analysis with a p-value of <0.25 were included in a backward logistic regression. Results are reported as adjusted OR with 95% CI, corrected for age, gender, weight-for-height z-score and culture-based bacterial carriage.
Results
General characteristics of participants
The majority of the population studied lived in an urban setting (392/414; 95%). The general characteristics of the study population is given in Table 1, anthropometric characteristics are given in Table 2. The study group had a mean age of 14.2±10 months, the youngest child being one month old, and the oldest 58 months. Fifty-eight percent (240/414) of the study population were boys.
Table 1. Description of socioeconomic characteristics (n = 414).
| Description of study population: socioeconomic status, sanitation | |
|---|---|
| Socio-economic status of family* | |
| Lowest income | 34/414 (8%) |
| Middle income | 331/414 (80%) |
| Highest income | 49/414 (12%) |
| Mother completed at least primary school | 224/414 (54%) |
| Father working | 318/414 (77%) |
| Drinking water source of children | |
| At least sometimes water from well | 81/414 (19%) |
| Running water or from fountain only | 222/414 (54%) |
| Only mineral water | 91/414 (22%) |
| Other (breastfeeding only etc.) | 20/414 (5%) |
| Drinking water source of family | |
| At least sometimes water from well | 239/414 (58%) |
| Running water or from fountain only | 174/414 (42%) |
| Only mineral water | 0/414 (0%) |
| Family treats water by chlorination | 128/414 (31%) |
| Eating with | |
| Fingers only | 113/414 (27%) |
| Cutlery only | 195/414 (47%) |
| Both | 97/414 (24%) |
| No data | 9/414 (2%) |
*defined as in Breurec et al., Plos Neglected Tropical Diseases 2015
Table 2. Description of general study population: Anthropometric measurements (n = 414).
| Description of study population: age, gender, anthropometric measurements | |
|---|---|
| Females | 122 /414 (46%) |
| Age of study population (months) 1 | 14.2 ±10.0 |
| HAZ of study population1, 2 | -1.45 ±1.7 |
| Stunted (based on HAZ) | |
| Normal growth (HAZ ≥ -2 SD) | 266/414 (64%) |
| Stunted (HAZ < -2 SD) | 148/414 (36%) |
| WHZ of study population1, 3 | 0.25 ± 1.31 |
| Acute malnutrition (based on WHZ) | |
| Normal weight (-2 SD≤ WHZ≤ 2SD) | 363/414 (88%) |
| Acute malnutrition (WHZ < -2 SD) | 16/414 (4%) |
| BMI z-score of study population1, 4 | 0.38 ±1.37 |
| Overweight (based on zBMI) | |
| Normal weight (-2 SD≤ zBMI≤ 2SD) | 355/414 (86%) |
| Overweight and obese (zBMI >2 SD) | 42/414 (10%) |
1 Mean ± Standard Deviation
2 stunted: < -2 height-for-age z-score (HAZ) (moderately stunted: -3 ≤ HAZ < -2; severely stunted: HAZ < -3)
3 acute malnutrition based on weight-for-height z-score (WHZ) score: < -2 WHZ (moderately acutely malnourished (MAM): -3 ≤ WHZ < -2; severely acutely malnourished (SAM): WHZ < -3).
4 Overweight based on Body-Mass Index z-score (zBMI): overweight: zBMI> 2SD (Overweight: 3≤ zBMI > 2 SD and obese zBMI > 3 SD)
Surprisingly, only 3% (14/414) of the parents co-habited; instead, most mothers were living with their children together with their extended family (397/414). Less than a third of the mothers declared themselves to be working (125/414). The median number of family members was 7 (interquartile range 5–10) and the median number of children below the age of 5 in each household was 2 (interquartile range 1–3). Eighty-eight percent (365/414) of all families had access to improved water, as defined by either running water or water from a public fountain. Seventy-six percent (313/414) of the children were given exclusively improved water for drinking. For one-fifth of the children (91/414), bottled mineral water was reported by the caregiver as the sole drinking water. It is noteworthy that the children received different water for drinking from that used by the family. Roughly a third of all families treated drinking water by chlorination (128/414).
Anthropometric characteristics of the participants
Thirty-six percent (148/414) of the study population showed stunted growth, of which roughly half showed a moderate delay (height-for-age z-score (HAZ) between -2 and -3 SD) and half a severe delay (HAZ < -3) in linear growth for their age group. Stunting was higher in boys, with 97/241 (40%) of all boys being stunted compared to 55/177 (31%) of girls. The stunted group had a higher median age (median 15.5 months, interquartile range: 11–22 months) than that observed for the non-stunted group (median 9 months, interquartile range: 7–13 months). The mean HAZ drastically decreases with increased age, averaging -0.89 HAZ (SD 1.44) for infants (0–11 months), -1.96 HAZ (SD 1.77) for toddlers (12–23 months) and -2.33 HAZ (SD 1.73) for children (≥ 24 months), while the mean weight-for-height z-score (WHZ) remained relatively stable (-0.46 (1.11 SD); -0.63 (1.17 SD); -1.2 (1.21 SD)) in infants, toddlers and children, respectively. The percentage of stunted children hence increased with age, progressing from 21% (45/218) in infants, 52% in toddlers (72/138) to 53% in children (31/58).
An analysis of the weight category, based the body weight index (zBMI), in relation to the stunting phenotype yielded the following: 356 of 414 children (86%) were of normal weight. Of these, 32% (120/372) were concurrently stunted. 16 of 414 children (4%) were underweight. Of these, 19% (3/16) were also concurrently stunted. In contrast, 42 of 414 (10%) were overweight, and 67% (28/42) of these were also concurrently stunted.
Thus, a striking percentage of overweight children were concurrently stunted. These overweight and concurrently stunted children accounted for 7% (28/414) of the entire study population,
Asymptomatic pathogen carriage as a risk factor for stunting
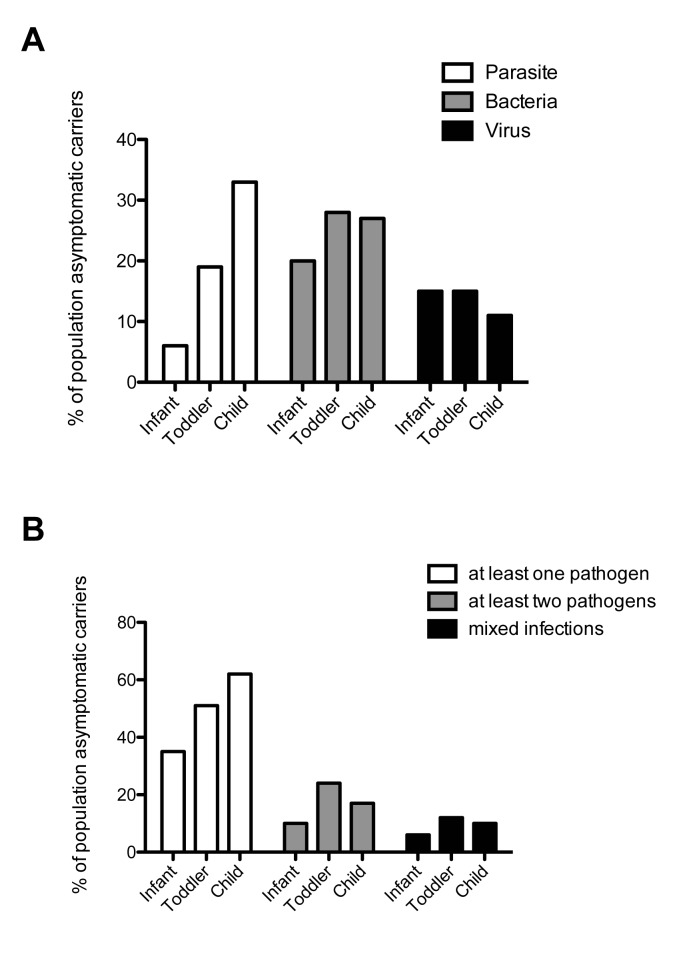
Overall asymptomatic pathogen carriage, as measured by conventional microbiological techniques, was generally low (see Table 3 and S2 Table for a full list). Only 14% (57/414) of the children tested positive for one or more parasites, 16% (61/414) of the children tested positive for one or more viruses, and only 23% (97/414) of children tested positive for a pathogenic bacterium. Of these bacterial pathogens, 9% (33/414) were culture positive. Cumulative, 44% (177/414) of children tested positive for at least one pathogen. Co-infection of at least two pathogens occurred in 16% (66/414) of children, while mixed infections of at least two groups of pathogens (bacteria, virus and/or parasite) occurred in 9% (37/414) of children. Asymptomatic pathogen carriage increased with age (see Fig 2). The most prevalent parasites, with about 1/10 children infected, were Cryptosporidium and Giardia, while only 1/50 children showed amoeba in their stools. Bacteria were more readily detected by PCR, as illustrated through the increased detection of Shigella in PCR compared to culture techniques. The most prevalent bacteria detected were Shigella and pathogenic E. coli (around 1/10 children), the most prevalent single E. coli strain, with around 1/20 carrier children, was enterotoxic E. coli (ETEC). About 1/20 children carried either rotavirus, norovirus, adenovirus or astrovirus (see S2 Table).
Table 3. Description of general study population for asymptomatic pathogen carriage (n = 414).
| Combined scores | |
| At least one pathogen detected | 177/414 (44%) |
| At least two pathogens detected | 66/414 (16%) |
| Mixed infection with at least two groups of pathogens (virus, parasites, bacterium) | 37/414 (9%) |
| Parasites | |
| At least one parasite detected by PCR or microscopy | 57/414 (14%) |
| Cryptosporidium parvum/hominis (PCR) | 39/414 (9%) |
| Giardia intestinalis (microscopy) | 32/414 (8%) |
| Amiba (microscopy) | 7/414 (2%) |
| Bacteria | |
| At least one bacterium detected by culture or PCR | 97/414 (23%) |
| At least one bacterium detected by culture | 33/414 (6%) |
| Shigella spp. (PCR) | 35/414 (8%) |
| Shigella spp. (culture) | 4/414 (1%) |
| Salmonella spp. (culture) | 13/414 (3%) |
| At least 1 pathogenic E. coli by PCR | 49/414 (12%) |
| ETEC | 19/414 (5%) |
| Virus | |
| At least one virus detected | 61/414 (16%) |
Fig 2. Pathogen load in different age categories.
A: Pathogen load by age category for the three main groups of pathogens, parasites (white bars), bacteria (grey bars) and viruses (black bars). B: Infection with multiple pathogens by age category. White bars indicate the presence of at least one pathogen of any group (parasite, bacteria or virus), grey bars the presence of at least two pathogens of any group (parasite, bacteria or virus) and black bars indicate mixed infections with at least one representative of two different groups (virus, parasite or bacteria) in the same child. Infant: 0–11 months, Toddler: 12–23 months; Child: ≥ 24 months).
There was a significant association between carriage of a parasite or a bacterium detected in culture and stunting in the univariate analysis (p = 0.022) (see Table 4). After adjustment for either age alone (data not shown) or age and gender (data not shown), no statistically significant association between parasite carriages and stunting was observed. A trend towards association between bacterial carriage and stunting was observed for the 9% of bacterial pathogens identified by culture methods, although the association remained non-significant (p = 0.069) (see Table 4). Notably, this trend is independent of age group as all age categories displayed an unadjusted OR of ~2, suggesting that significance might not have been reached due to a limited sample size.
Table 4. Risk factors associated with stunting (n = 414).
| Non stunted | Stunted | Unadjusted OR | p-value unadjusted | Adjusted OR | p-value adjusted OR* | |
|---|---|---|---|---|---|---|
| N = 266 | N = 148 | (95%CI) | OR | (95%CI)* | ||
| General factors | ||||||
| Females | 122 (46%) | 52 (35%) | 0.64 (0.42; 0.97) | 0.034 | 0.61 (0.38; 0.94) | 0.027 |
| Age (months)1 | 12.2 ± 9.5 | 17.6 ±10.1 | p< 0.0001 | p< 0.0001 | ||
| Infant | 173 (65%) | 45 (30%) | 1 | 1 | ||
| Toddler | 66 (25%) | 72 (49%) | 4.19 (2.63; 6.70) | 3.98 (2.45; 6.46) | ||
| Child | 27 (10%) | 31 (21%) | 4.41 (2.40; 8.13) | 4.42 (2.36; 8.28) | ||
| Weight-for-height z-score (WHZ)1 | 0.08 ±1.26 | 0.55 ±1.34 | 0.003 | 0.009 | ||
| Normal | 239 (90%) | 124 (84%) | 1 | 1 | ||
| MAM/SAM | 13 (5%) | 3 (2%) | 0.44 (0.12; 1.59) | 0.67 (0.18; 2.54) | ||
| Overweight | 14 (5%) | 21 (14%) | 2.89 (1.42; 5.88) | 3.21 (1.50; 6.90) | ||
| Sanitation and socio-economic factors | ||||||
| Water source of child | 0.010 | 0.498 | ||||
| At least sometimes water from well | 46 (17%) | 35 (24%) | 1 | |||
| Running water or from fountain only | 136 (51%) | 86 (58%) | 0.35 (0.18; 0.68) | |||
| Only pure water | 72 (27%) | 19 (13%) | 0.83 (0.50; 1.39) | |||
| Other (breastfeeding etc.) | 12 (5%) | 8 (5%) | 0.88 (0.32; 2.37) | |||
| Eating with | 0.0005 | 0.741 | ||||
| Fingers only | 60 (23%) | 53 (37%) | 1 | |||
| Cutlery only | 144 (55%) | 51 (35%) | 2.07 (1.24; 3.46) | |||
| Both | 56 (22%) | 41 (28%) | 2.49 (1.53; 4.06) | |||
| Mother lives with family | 258 (97%) | 139 (94%) | 0.139 | 0.175 | ||
| Mother completed at least primary school | 141 (53%) | 83 (56%) | 0.508 | 0.526 | ||
| Socio-economic score2 | 0.346 | 0.085 | ||||
| Lowest income | 19 (7%) | 15 (10%) | 1 | 1 | ||
| Middle income | 212 (80%) | 119 (80%) | 0.71 (0.35; 1.45) | 0.55 (0.25; 1.23) | ||
| Highest income | 35 (13%) | 14 (10%) | 0.51 (0.20; 1.27) | 0.31 (0.11; 0.88) | ||
| Asymptomatic pathogen carriage | ||||||
| Pathogen found | 106 (40%) | 71 (49%) | 0.119 | 0.805 | ||
| (Parasite, Bacterium or Virus) | ||||||
| Parasite found | 29 (11%) | 28 (19%) | 1.91 (1.09; 3.35) | 0.025 | 0.370 | |
| Giardia lamblia | 17 (6%) | 15 (10%) | 0.175 | 0.801 | ||
| Virus found (PCR) | 41 (15%) | 20 (14%) | 0.384 | 0.390 | ||
| Rotavirus (PCR) | 13 (5%) | 3 (2%) | 0.151 | 0.148 | ||
| Noroviurs (PCR) | 11 (4%) | 3 (2%) | 0.253 | 0.253 | ||
| Bacterium in culture found | 17 (6%) | 18 (12%) | 2.32 (1.13; 4.75) | 0.022 | 2.04 (0.95; 4.41) | 0.069 |
| Salmonella spp. | 6 (2%) | 7 (5%) | 0.176 | 0.586 | ||
| ETEC | 8 (3%) | 11 (7%) | 2.59 (1.02; 6.59) | 0.046 | 0.299 | |
* OR adjusted for age category, gender, weight-for-height z-score, positive for bacterial culture
1 Mean ± standard deviation
2 as described in Breurec et al., PNTD 2016
Other risk factors for stunting
Table 4 shows the prevalence of stunting and its associated risk factors. In a univariate analysis, several factors indicating hygiene status. For example, providing bottled mineral water as the child’s sole water source or using cutlery for eating were associated with less stunting. However, the association disappeared when adjusted for age. Education of mother and size of the household were not significantly associated with stunting. Socio-economic status was not significantly associated (p = 0.085), showed however a trend to protection as the socio-economic status rose with an aOR of 0.55 (95% CI: 0.25; 1.23) from lowest income to middle income and an aOR of 0.31 (95% CI: 0.11; 0.88) from lowest to highest income (see Table 4). Using logistic regression, the only three variables independently associated with stunting were gender, age and being overweigh. Boys showed an approximately 1.7x higher risk than girls of being stunted (aOR: 1.67; 95% CI: 1.07; 2.62). Toddlers (12–23 months) were about 4-fold (aOR 3.98; 95% CI: 2.45; 6.46) more likely to be stunted compared to infants (0–11 months), while children (24–59 months) were about 4.5-fold (aOR 4.42; 95% CI: 2.36; 8.28) more likely to be stunted than infants. In addition, overweight children were roughly three times more likely to be stunted (aOR: 3.21; 95% CI: 1.50; 6.90) compared to normal weight children.
Description of the stunted-overweight population
Stunted children showed a more than 4-fold increase (aOR: 4.2; 95% CI: 2.13; 8.27) in the risk of being overweight. Around 7% (28/414) of the total study population was concurrently stunted and overweight (stunted-overweight). The characteristics of this population, as well as the stunted-only and overweight-only populations, is given in S3 Table. Similar to the entire stunted population, the risk of the stunted-overweight phenotype increased significantly with age (OR 3.48, 95% CI: 1.44, 8.36 in toddlers compared to infants; OR 3.5, 95% CI: 1.21, 10.08 in children compared to infants). No other significant associations with potential risk factors were identified.
Discussion
This cross-sectional study analysed 414 children aged five years and under living in Bangui for risk factors associated with stunting. The children were recruited from December 2011 until November 2013, and thus before the outbreak of the 2013–16 civil war. After correction for age, we found no significant statistical association between asymptomatic pathogen carriage and stunting. However, an increased risk of stunted children concurrently being overweight was observed. This is one of the first studies in the CAR looking at risk factors associated with stunting and the first study to link stunting with a much higher increased risk of being overweight in one of the least developed countries of the world. Indeed, stunted children were much more likely to be overweight than underweight. Given this finding, nutritional supplementation to ameliorate the stunting phenotype, which is believed to be, at least in part, a manifestation of chronic undernutrition, need to be carefully designed and monitored in order to prevent exposing the stunted children to the additional health risks that are associated with being overweight.
Our study has however several weaknesses: first and foremost, stunting is a gradual process, emerging from a long-term chronic undernutrition and/or repeated infections (reviewed in [6]). It would therefore be better to analyse risk factors in a longitudinal study, in order to be able to draw causal conclusions. The study also did not include a large number of children, which made it impossible to further stratify the children based on age groups. As infants and toddlers experience a different environment than older children (e.g. eating with hands, receiving special drinking water and other foods), it would be interesting to be able to analyse the associated risk factors of stunting in the three age groups independently in future studies.
Molecular techniques were more sensitive to detect low levels of pathogens, as illustrated through the carriage of Shigella, which was assessed through both culture and PCR. It would therefore be interesting to use more sensitive techniques in future studies to look at asymptomatic pathogen carriage. However, given the long list of pathogens tested, our study contributes valuable insights into the possible associations between asymptomatic pathogen carriage and stunting. It also highlights the need to adjust for age when analysing the association in cross-sectional studies as pathogen carriage was associated with stunting in a univariate analysis but disappeared once adjusted for age.
Our analysis revealed age, gender and being overweight to be the most significant risk factors associated with stunting in young children living in the CAR. In a meta-study that consolidated 18 separate studies from Sub-Saharan Africa, the main factors associated with stunting were gender (boys being more stunted than girls), socio-economic factors and maternal education [37]. Another meta-analysis of 16 demographic and health surveys from Sub-Saharan Africa showed that boys of socio-economically weaker families were at greater risk of experiencing stunted growth than girls [38]. In accordance with these findings, our data identified boys to be at a higher risk than girls of being stunted. To date, it is unclear what social or biological reason might account for this difference. Our study did not find any association of stunted growth with socio-economic status or maternal education. This could be for several reasons: most of the families were living in similar conditions, which did not permit detection of a possible effect of socio-economic status on growth. Furthermore, because the study was conducted in the capital, formal education might have less influence on health, nutrition and sanitation choices than it would have in a rural setting, where access to information is more difficult.
Children in low-income countries are prone to helminth infections (see as an example [28,39–41], a phenomenon suspected to be potentially associated with chronic undernutrition [11,42]. It is surprising that no helminths were found in the stool specimens given the high burden of chronic malnutrition, the still wide-spread use of non-improved well water by 239 of the 414 families (58%) and the socio-economic challenges facing the people of the CAR,. This observation could be due to the frequent use of mebendazole, a drug that is acting mainly against helminthic infections and is widely available and used by mothers in Bangui to treat their children. In addition, mebendazole is given to children through mass deworming campaigns by non-governmental organizations. For example, in 2013, 417,898 children aged one to five years received mebendazole in CAR through a UNICEF program (https://www.unicef.org/appeals/files/HAC_2014_CAR_-_Revised_06-05-2014.pdf). More in depth data on this question would be needed in order to conclusively explain this phenomenon and to investigate the impact this will have on the health of these children.
Our analysis revealed that 7% of all children in the study group were concurrently stunted and overweight. This phenomenon was described as early as 1996 by Popkin and collaborators [43] among children aged 3–6 and 7–9 years in national surveys in Russia, Brazil, the Republic of South Africa and China, four countries entering a nutritional transition at this time. The risk ratios of being overweight for stunted children ranged from 1.7 to 7.8 in these studies. This observation gained momentum again in the last years due to the alarming global increase in childhood obesity and the particularly dramatic upsurge of obesity in developing countries [44]. Stunted preschool children were shown to have an increased risk to be overweight in South Africa (the Limpopo province)[45], Cameroon [46], Brazil [47], rural Mexico [48], Guatemala [49], Uruguay, Ecuador [50,51], China [52] and Indonesia [53]. Prevalence of concurrent stunting and being overweight ranged in these studies between 2.5% in China [52] to over 19% in the study conducted in the Limpopo region of South Africa [45]. There are several hypotheses that could explain the seemingly paradox nature of the co-existence of these two phenomenon. The first hypothesis, proposed by Mamabolo and colleagues [45], contends that poor food quality is to blame. Such food is typically low in animal proteins, micronutrients and fat and rich in carbohydrates. The authors hypothesize that the low protein and fat content leads to linear growth deficits while the high carbohydrate content leads to increased fat mass. Another hypothesis is the so-called Barker hypothesis, which emerged from observations during the Dutch Famine of the Second World War. These observations showed that men suffering from food deprivation during the first half of gestation had a higher risk of obesity at age 19 [54]. Further work suggested that food deprivation early on in life can lead to metabolic changes that pre-dispose for obesity and metabolic disease later in life [55–58]. A proposed explanation for the Barker hypothesis is that in contrast to normal weight gain, malnourished children have a disequilibrium in growth hormone and other growth factors, leading them to develop a higher proportion of fat and lower proportion of lean tissue. This explanation was supported in a study assessing the body composition in 20 stunted versus 30 healthy children aged 11 to 15 years and living in the slums of Sao Paulo, Brazil [59]. Furthermore, in a longitudinal study among school girls followed for two years, stunted girls gained more weight when exposed to a high fat diet compared to their non-stunted colleagues and also had higher central fat accumulation as reflected through a lower waist-to-hip ratio [60]. The same observation was made in another study where children were followed for over four years [61]. A study of 58 pre-pubertal adolescents also showed that fasting beta oxidation of fatty acids was decreased in stunted compared to healthy children living in a shantytown of Sao Paulo [62], while their resting energy expenditure remained normal [63]. Hence, the decreased ability to break down fatty acids to Acetyl-CoA while not changing the amount of energy used in the body in general could lead to enhanced presence of fatty acids and hence possibly leading to deposition of the fatty acids as stored fat tissue. The same investigators also showed that in a group of 56 children aged 8–11 years living in the shantytown of Sao Paulo, the regulation of energy intake was impaired in stunted children compared to the normally nourished control group, suggesting that part of the phenomenon could also be due to “opportunistic overeating” by the stunted children leading to excessive food intake and hence overweight [64].
More data is needed in order to really understand the metabolic mechanisms leading to the phenomenon of the concurrent occurrence of stunting and being overweight (termed stunting-overweight).
Our study is clearly underpowered to look at risk factors associated with this phenomenon. More studies in the CAR are needed to shed light on the precise risk factors and mechanisms underlying stunting-overweight.
As the CAR is one of the poorest countries in the world, our study reveals for the first time that stunting-overweight is no longer a problem of richer affluent communities such as Brazil, China or Indonesia undergoing large-scale nutritional transition, but represents a major public health risk for the entire developing world in the coming decades.
More data is needed in order to consolidate the findings described in this paper. However, the co-occurrence of stunting and overweight in one of the poorest countries of the world is alarming and, if the results are confirmed in further studies, the increased risk of stunted children for obesity should be considered when designing treatment schemes for chronically malnourished children.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLS)
Acknowledgments
We would like to thank the children and parents that participated in the initial study as well as the staff from the Complexe Pédiatrique de Bangui and the Institut Pasteur in Bangui, particularly Ernest José Gbao and Natacha Zato, the two field investigators of the original study. We also would like to thank André Briend and Pamela Schnupf for helpful discussion of the results and Pamela Schnupf for careful reading of the manuscript.
Data Availability
Anonymised, relevant data are within the paper and its Supporting Information files.
Funding Statement
The original study was funded by the Total Foundation. Pascale Vonaesch is the recipient of a SNF Early. Postdoc Mobility Fellowship as well as a Roux Cantarini Postdoctoral Fellowship. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Adair LS. Long-term consequences of nutrition and growth in early childhood and possible preventive interventions. Nestle Nutr Inst Workshop Ser. 2014;78:111–20. doi: 10.1159/000354949 [DOI] [PubMed] [Google Scholar]
- 2.Adair LS. Developing world perspective: the importance of growth for short-term health. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:71–9–discussion79–83. doi: 10.1159/000281146 [DOI] [PubMed] [Google Scholar]
- 3.Mata LJ, Kromal RA, Urrutia JJ, Garcia B. Effect of infection on food intake and the nutritional state: perspectives as viewed from the village. Am J Clin Nutr. 1977. August;30(8):1215–27. [DOI] [PubMed] [Google Scholar]
- 4.Guerrant RL, Oriá RB, Moore SR, Oriá MOB, Lima AAM. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008. September;66(9):487–505. doi: 10.1111/j.1753-4887.2008.00082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011. October;7 Suppl 3:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014. November;34(4):250–65. doi: 10.1179/2046905514Y.0000000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald CM, Olofin I, Flaxman S, Fawzi WW, Spiegelman D, Caulfield LE, et al. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr. 2013. April;97(4):896–901. doi: 10.3945/ajcn.112.047639 [DOI] [PubMed] [Google Scholar]
- 8.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010. March;125(3):e473–80. doi: 10.1542/peds.2009-1519 [DOI] [PubMed] [Google Scholar]
- 9.de Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr. 2016. May;12 Suppl 1:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008. April;4 Suppl 1:24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001. December;30(6):1457–64. [DOI] [PubMed] [Google Scholar]
- 12.Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barrett LJ, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010. October;139(4):1156–64. doi: 10.1053/j.gastro.2010.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima AA, Moore SR, Barboza MS, Soares AM, Schleupner MA, Newman RD, et al. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis. 2000. May;181(5):1643–51. doi: 10.1086/315423 [DOI] [PubMed] [Google Scholar]
- 14.Guerrant RL, Kirchhoff LV, Shields DS, Nations MK, Leslie J, de Sousa MA, et al. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis. 1983. December;148(6):986–97. [DOI] [PubMed] [Google Scholar]
- 15.Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008. April;118(4):1277–90. doi: 10.1172/JCI34005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira D, Ferreira FS, Atouguia J, Fortes F, Guerra A, Centeno-Lima S. Infection by Intestinal Parasites, Stunting and Anemia in School-Aged Children from Southern Angola. PLoS ONE. 2015;10(9):e0137327 doi: 10.1371/journal.pone.0137327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima AA, Fang G, Schorling JB, de Albuquerque L, McAuliffe JF, Mota S, et al. Persistent diarrhea in northeast Brazil: etiologies and interactions with malnutrition. Acta Paediatr Suppl. 1992. September;381:39–44. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy G, Nantel G, Brouwer ID, Kok FJ. Does living in an urban environment confer advantages for childhood nutritional status? Analysis of disparities in nutritional status by wealth and residence in Angola, Central African Republic and Senegal. Public Health Nutr. 2006. April;9(2):187–93. [DOI] [PubMed] [Google Scholar]
- 19.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008. January 19;371(9608):243–60. doi: 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- 20.Caulfield LE, Richard SA, Black RE. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. Am J Trop Med Hyg. 2004. August;71(2 Suppl):55–63. [PubMed] [Google Scholar]
- 21.Costa LB, JohnBull EA, Reeves JT, Sevilleja JE, Freire RS, Hoffman PS, et al. Cryptosporidium-malnutrition interactions: mucosal disruption, cytokines, and TLR signaling in a weaned murine model. J Parasitol. 2011. December;97(6):1113–20. doi: 10.1645/GE-2848.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolick DT, Roche JK, Hontecillas R, Bassaganya-Riera J, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli strain in a novel weaned mouse model: exacerbation by malnutrition, biofilm as a virulence factor and treatment by nitazoxanide. J Med Microbiol. 2013. June;62(Pt 6):896–905. doi: 10.1099/jmm.0.046300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J Infect Dis. 2010. August 15;202(4):506–14. doi: 10.1086/654894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartelt LA, Roche J, Kolling G, Bolick D, Noronha F, Naylor C, et al. Persistent G. lamblia impairs growth in a murine malnutrition model. J Clin Invest. 2013. June;123(6):2672–84. doi: 10.1172/JCI67294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown EM, Wlodarska M, Willing BP, Vonaesch P, Han J, Reynolds LA, et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat Commun. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved; 6 SP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G, Paredes Olortegui M, Peñataro Yori P, Black RE, Caulfield L, Banda Chavez C, et al. Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatr Infect Dis J. 2014. October;33(10):1004–9. doi: 10.1097/INF.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 27.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998. September 1;148(5):497–506. [DOI] [PubMed] [Google Scholar]
- 28.LaBeaud AD, Nayakwadi Singer M, McKibben M, Mungai P, Muchiri EM, McKibben E, et al. Parasitism in Children Aged Three Years and Under: Relationship between Infection and Growth in Rural Coastal Kenya. PLoS Negl Trop Dis. 2015. May;9(5):e0003721 doi: 10.1371/journal.pntd.0003721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013. July 20;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 30.Breurec S, Vanel N, Bata P, Chartier L, Farra A, Favennec L, et al. Etiology and Epidemiology of Diarrhea in Hospitalized Children from Low Income Country: A Matched Case-Control Study in Central African Republic. PLoS Negl Trop Dis. 2016. January;10(1):e0004283 doi: 10.1371/journal.pntd.0004283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randremanana RV, Razafindratsimandresy R, Andriatahina T, Randriamanantena A, Ravelomanana L, Randrianirina F, et al. Etiologies, Risk Factors and Impact of Severe Diarrhea in the Under-Fives in Moramanga and Antananarivo, Madagascar. PLoS ONE. 2016;11(7):e0158862 doi: 10.1371/journal.pone.0158862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosek MN, MAL-ED Network Investigators. Causal Pathways from Enteropathogens to Environmental Enteropathy: Findings from the MAL-ED Birth Cohort Study. EBioMedicine. 2017. April;18:109–17. doi: 10.1016/j.ebiom.2017.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Group WMGRS. … Standards: Head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: Methods and development Geneva: World Health …; 2007. [Google Scholar]
- 34.Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta paediatrica. 2006. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman DJ, Sawaya AL, Martins PA, McCrory MA, Roberts SB. Comparison of techniques to evaluate adiposity in stunted and nonstunted children. Pediatrics. 2006. April;117(4):e725–32. doi: 10.1542/peds.2005-0779 [DOI] [PubMed] [Google Scholar]
- 36.Sire J-M, Garin B, Chartier L, Fall NK, Tall A, Seck A, et al. Community-acquired infectious diarrhoea in children under 5 years of age in Dakar, Senegal. Paediatr Int Child Health. 2013. August;33(3):139–44. doi: 10.1179/2046905512Y.0000000046 [DOI] [PubMed] [Google Scholar]
- 37.Keino S, Plasqui G, Ettyang G, van den Borne B. Determinants of stunting and overweight among young children and adolescents in sub-Saharan Africa. Food Nutr Bull. 2014. June;35(2):167–78. doi: 10.1177/156482651403500203 [DOI] [PubMed] [Google Scholar]
- 38.Wamani H, Astrøm AN, Peterson S, Tumwine JK, Tylleskär T. Boys are more stunted than girls in sub-Saharan Africa: a meta-analysis of 16 demographic and health surveys. BMC Pediatr. 2007;7:17 doi: 10.1186/1471-2431-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilechukwu G, Ilechukwu C, Ubesie A, Onyire N, Emechebe G, Eze J. Relationship between nutritional status and intensity of common intestinal helminths among children in enugu, South-East Nigeria. Ann Med Health Sci Res. 2014. July;4(Suppl 2):S119–22. doi: 10.4103/2141-9248.138027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anuar TS, Salleh FM, Moktar N. Soil-transmitted helminth infections and associated risk factors in three Orang Asli tribes in Peninsular Malaysia. Sci Rep. 2014. February 14;4:4101 doi: 10.1038/srep04101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngui R, Aziz S, Chua KH, Aidil RM, Lee SC, Tan TK, et al. Patterns and Risk Factors of Soil-Transmitted Helminthiasis Among Orang Asli Subgroups in Peninsular Malaysia. Am J Trop Med Hyg. 2015. August;93(2):361–70. doi: 10.4269/ajtmh.13-0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papier K, Williams GM, Luceres-Catubig R, Ahmed F, Olveda RM, McManus DP, et al. Childhood malnutrition and parasitic helminth interactions. Clin Infect Dis. 2014. July 15;59(2):234–43. doi: 10.1093/cid/ciu211 [DOI] [PubMed] [Google Scholar]
- 43.Popkin BM, Richards MK, Montiero CA. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J Nutr. 1996. December;126(12):3009–16. [DOI] [PubMed] [Google Scholar]
- 44.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010. November;92(5):1257–64. doi: 10.3945/ajcn.2010.29786 [DOI] [PubMed] [Google Scholar]
- 45.Mamabolo RL, Alberts M, Steyn NP, Delemarre-van de Waal HA, Levitt NS. Prevalence and determinants of stunting and overweight in 3-year-old black South African children residing in the Central Region of Limpopo Province, South Africa. Public Health Nutr. 2005. August;8(5):501–8. [DOI] [PubMed] [Google Scholar]
- 46.Said-Mohamed R, Allirot X, Sobgui M, Pasquet P. Determinants of overweight associated with stunting in preschool children of Yaounde, Cameroon. Ann Hum Biol. 2009. March;36(2):146–61. doi: 10.1080/03014460802660526 [DOI] [PubMed] [Google Scholar]
- 47.Florêncio TM, Ferreira HS, de França AP, Cavalcante JC, Sawaya AL. Obesity and undernutrition in a very-low-income population in the city of Maceió, northeastern Brazil. Br J Nutr. 2001. August;86(2):277–84. [DOI] [PubMed] [Google Scholar]
- 48.Fernald LC, Neufeld LM. Overweight with concurrent stunting in very young children from rural Mexico: prevalence and associated factors. Eur J Clin Nutr. 2007. May;61(5):623–32. doi: 10.1038/sj.ejcn.1602558 [DOI] [PubMed] [Google Scholar]
- 49.Ramirez-Zea M, Kroker-Lobos MF, Close-Fernandez R, Kanter R. The double burden of malnutrition in indigenous and nonindigenous Guatemalan populations. Am J Clin Nutr. 2014. December;100(6):1644S–51S. doi: 10.3945/ajcn.114.083857 [DOI] [PubMed] [Google Scholar]
- 50.Bove I, Miranda T, Campoy C, Uauy R, Napol M. Stunting, overweight and child development impairment go hand in hand as key problems of early infancy: Uruguayan case. Early Hum Dev. 2012. September;88(9):747–51. doi: 10.1016/j.earlhumdev.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 51.Freire WB, Silva-Jaramillo KM, Ramírez-Luzuriaga MJ, Belmont P, Waters WF. The double burden of undernutrition and excess body weight in Ecuador. Am J Clin Nutr. 2014. December;100(6):1636S–43S. doi: 10.3945/ajcn.114.083766 [DOI] [PubMed] [Google Scholar]
- 52.Piernas C, Wang D, Du S, Zhang B, Wang Z, Su C, et al. The double burden of under- and overnutrition and nutrient adequacy among Chinese preschool and school-aged children in 2009–2011. Eur J Clin Nutr. 2015. December;69(12):1323–9. doi: 10.1038/ejcn.2015.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rachmi CN, Agho KE, Li M, Baur LA. Stunting coexisting with overweight in 2·0–4·9-year-old Indonesian children: prevalence, trends and associated risk factors from repeated cross-sectional surveys. Public Health Nutr. 2016. October;19(15):2698–707. doi: 10.1017/S1368980016000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976. August 12;295(7):349–53. doi: 10.1056/NEJM197608122950701 [DOI] [PubMed] [Google Scholar]
- 55.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006. February;46(1):4–14. doi: 10.1111/j.1479-828X.2006.00506.x [DOI] [PubMed] [Google Scholar]
- 56.Langley-Evans SC. Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet. 2015. January;28 Suppl 1:1–14. [DOI] [PubMed] [Google Scholar]
- 57.Joseph KS, Kramer MS. Review of the evidence on fetal and early childhood antecedents of adult chronic disease. Epidemiol Rev. 1996;18(2):158–74. [DOI] [PubMed] [Google Scholar]
- 58.Ozanne SE. Metabolic programming in animals. Br Med Bull. 2001;60:143–52. [DOI] [PubMed] [Google Scholar]
- 59.Martins PA, Hoffman DJ, Fernandes MTB, Nascimento CR, Roberts SB, Sesso R, et al. Stunted children gain less lean body mass and more fat mass than their non-stunted counterparts: a prospective study. Br J Nutr. 2004. November;92(5):819–25. [DOI] [PubMed] [Google Scholar]
- 60.Sawaya AL, Grillo LP, Verreschi I, da Silva AC, Roberts SB. Mild stunting is associated with higher susceptibility to the effects of high fat diets: studies in a shantytown population in São Paulo, Brazil. J Nutr. 1998. February;128(2 Suppl):415S–420S. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman DJ, Martins PA, Roberts SB, Sawaya AL. Body fat distribution in stunted compared with normal-height children from the shantytowns of São Paulo, Brazil. Nutrition. 2007. September;23(9):640–6. doi: 10.1016/j.nut.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 62.Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from São Paulo, Brazil. Am J Clin Nutr. 2000. September;72(3):702–7. [DOI] [PubMed] [Google Scholar]
- 63.Hoffman DJ, Sawaya AL, Coward WA, Wright A, Martins PA, de Nascimento C, et al. Energy expenditure of stunted and nonstunted boys and girls living in the shantytowns of São Paulo, Brazil. Am J Clin Nutr. 2000. October;72(4):1025–31. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman DJ, Roberts SB, Verreschi I, Martins PA, de Nascimento C, Tucker KL, et al. Regulation of energy intake may be impaired in nutritionally stunted children from the shantytowns of São Paulo, Brazil. J Nutr. 2000. September;130(9):2265–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLS)
Data Availability Statement
Anonymised, relevant data are within the paper and its Supporting Information files.