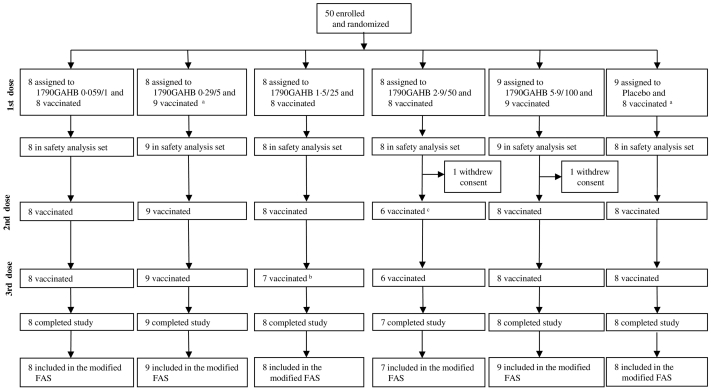
Fig. 1.
Study 1 - H03_01TP trial profile.
aOne subject was randomized to receive placebo but he/she received 3 vaccinations of 0.29/5 μg S. sonnei vaccine.
bOne subject received vaccine forbidden by the protocol and didn't receive third vaccination.
cOne subject developed neutropenia after the first vaccination and he/she didn't receive the following vaccinations.
FAS = full analysis set. The analysis of immunogenicity was based on modified FAS defined as: all randomized subjects who received at least one study vaccination and provided immunogenicity data at relevant time points. Subjects who received wrong vaccine at all vaccinations were analysed in the vaccine the subject actually received and blood samples collected after a vaccination visit but vaccine was not administered, were excluded from the analysis.