Fig 1. Qb VLP and PD vaccine preparation.
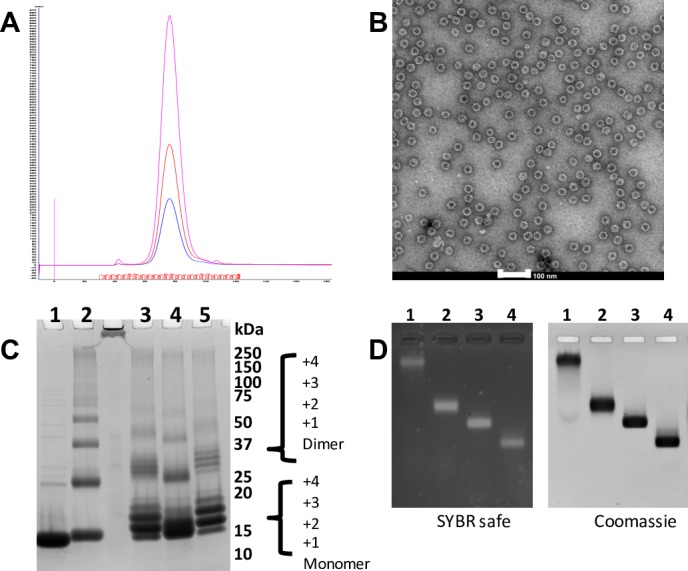
(A) Qb positive fractions from anion exchange were pooled and concentrated prior to size exclusion chromatography (SEC) on 16/600 Sephacryl S500-HR column, elution monitored by UV at 280 nm (blue), 254 nm (red) and 220 nm (pink). (B) A sample from the major SEC peak (adjusted to 0.1 mg/mL) was negatively stained and viewed by TEM (scale bar, 100nm). (C) Coomassie stained SDS-PAGE of peptide conjugated VLP preparation of PD vaccines. Purified VLPs (lane 1), derivatised with SMPH (lane 2) and subsequent conjugation with peptides PD1 (lane 3), PD2 (lane 4) and PD3 (lane 5) (Precision Plus Protein Standards, Bio-Rad. Sizes in kDa as indicated). (D) Vaccine preparations loaded on native agarose gel were stained for nucleic acid with SYBR safe (left) and for protein with Coomassie (right). Purified VLPs (lane 1), derivatised with SMPH (lane 2), conjugated with peptides PD1 (lane 3) and PD3 (lane 4).
