Abstract
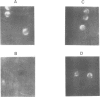
Mouse teratocarcinoma cells express neither H-2 heavy chains nor beta 2-microglobulin (beta 2-m). We have constructed two genomic libraries, one from PCC4-aza-RI embryonal carcinoma cells and the other from their adult syngenic counterpart 129/Sv liver cells (H-2bc). The libraries were screened with a full length mouse beta 2-m cDNA probe which we isolated and sequenced. Two cosmid clones carrying the entire beta 2-m gene were isolated, one from each library. There was no detectable difference in structure between the two genes. Furthermore, both were shown to be active and to restore beta 2-m synthesis upon transfer into mutant cells deficient in beta 2-m. Irreversible DNA alterations in or around the beta 2-m gene are thus unlikely to account for the lack of beta 2-m gene expression in embryonal teratocarcinoma cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg P. Dissections and reconstructions of genes and chromosomes. Science. 1981 Jul 17;213(4505):296–303. doi: 10.1126/science.6264595. [DOI] [PubMed] [Google Scholar]
- Buc-Caron M. H., Condamine H., Jacob F. The presence of F9 antigen on the surface of mouse embryonic cells until day 8 of embryogenesis. J Embryol Exp Morphol. 1978 Oct;47:149–160. [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Huebner K., Parnes J. R., Margulies D. H., Appella E., Seidman J. G. Control of expression of histocompatibility antigens (H-2) and beta 2-microglobulin in F9 teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5754–5758. doi: 10.1073/pnas.78.9.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois P., Fellous M., Gachelin G., Jacob F., Kemler R., Pressman D., Tanigaki N. Absence of a serologically detectable association of murine beta2-microglobulin with the embryonic F9 antigen. Transplantation. 1976 Nov;22(5):467–473. doi: 10.1097/00007890-197611000-00009. [DOI] [PubMed] [Google Scholar]
- Gachelin G. The cell surface antigens of mouse embryonal carcinoma cells. Biochim Biophys Acta. 1978 Sep 18;516(1):27–60. doi: 10.1016/0304-419x(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Lalanne J. L., Delarbre C., Gachelin G., Kourilsky P. A cDNA clone containing the entire coding sequence of a mouse H-2Kd histocompatibility antigen. Nucleic Acids Res. 1983 Mar 11;11(5):1567–1577. doi: 10.1093/nar/11.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Cunningham B. A., Jazwinski S. M., Hopp T. P., Blobel G., Edelman G. M. Cell-free synthesis and segregation of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3651–3655. doi: 10.1073/pnas.76.8.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Morello D., Gachelin G., Dubois P., Tanigaki N., Pressman D., Jacob F. Absence of reaction of a xenogenic anti-H-2 serum with mouse embryonal carcinoma cells. Transplantation. 1978 Aug;26(2):119–125. doi: 10.1097/00007890-197808000-00012. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Natori T., Katagiri M., Tanigaki N., Pressman D. The 11,000-dalton component of mouse H-2. Isolation and identification. Transplantation. 1974 Dec;18(6):550–555. doi: 10.1097/00007890-197412000-00015. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
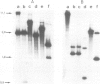
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Binetruy B., Cuzin F. High frequency of gene transfer after fusion between bacteria and eukaryotic cells. Nature. 1982 Jan 21;295(5846):257–259. doi: 10.1038/295257a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sawicki J. A., Magnuson T., Epstein C. J. Evidence for expression of the paternal genome in the two-cell mouse embryo. Nature. 1981 Dec 3;294(5840):450–451. doi: 10.1038/294450a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]