Abstract
The role of cardiac computed tomography (CT) for evaluating the mitral valve (MV) has been limited since echocardiography is the main method of evaluation. However, recent advances in cardiac CT have enable detailed evaluation of the anatomy and geometry of the MV. We describe assessments of the anatomy and coaptation geometric parameters of normal MVs, and also review repair of diseased/damaged MV. We also discuss pre- and post-surgical imaging of MV pathology using cardiac CT and various CT images. We found that cardiac CT could be used as an alternative imaging modality to echocardiography for pre-operative MV evaluation and to predict clinical outcomes following repair.
Keywords: Mitral valve, Cardiac valve, Cardiac computed tomography, CT, Mitral valve regurgitation, Mitral valve repair, Mitral regurgitation
INTRODUCTION
Mitral regurgitation (MR) is a heart valve disorder wherein the mitral valve (MV) does not close adequately when the heart pumps blood. In such cases, MR or severe leakage from a damaged structure, will necessitate heart surgery to repair or replace the valve. The underlying mechanism for MV disease and surgical outcomes are not clear. Therefore, to know the normal anatomy and coaptation geometry of the MV is essential for understanding the changes in MV before and after surgery.
Transthoracic echocardiography is the main evaluation method for the MV. However, echocardiography has a few limitations that depend on the operators, patients, and instrument. In these cases, cardiac computed tomography (CT) can provide additional detailed anatomic information. Although CT is primarily performed to evaluate coronary artery disease, it may be useful in planning and assessing MV repair.
Comparisons of three-dimensional (3D) transesophageal echocardiography and cardiac CT in the literature have shown that both procedure provided excellent visualization of MV morphology and geometry (1,2). Recent advances in 3D and four-dimensional (4D) imaging techniques for cardiac CT have enabled detailed visualizations of MV anatomy, complexity, and dynamics. Furthermore, these advances have enabled the development of valuable guidelines for the surgical treatment of functional MR by using cardiac CT (3,4). In this review, we describe assessments of the anatomy and coaptation geometric parameters of normal MVs and surgical advances in MV reconstruction together with the development of new technologies, and discuss the clinical usefulness of the pre- and post-operative CT images for the surgical planning and follow-up of the MV.
MV Anatomy
The MV is a dual-flap valve that is critical for the unidirectional blood flow through the heart (5). The main components of the MV apparatus are the mitral annulus, MV leaflets, chordae tendineae, and papillary muscles (Figs. 1, 2) (5,6,7).
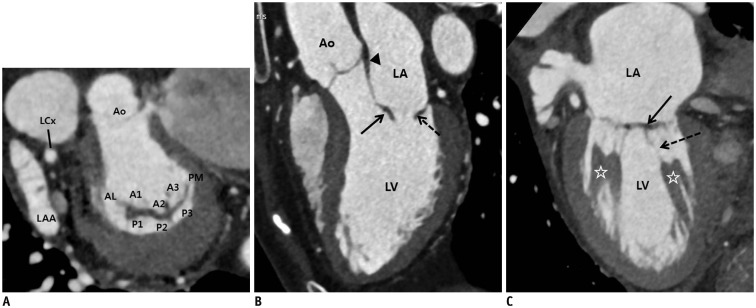
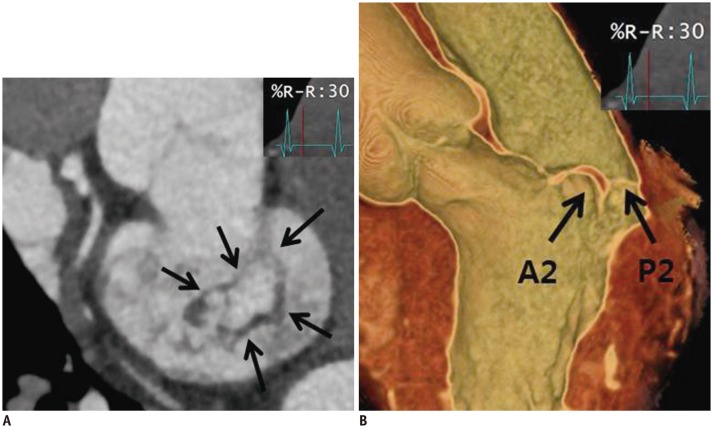
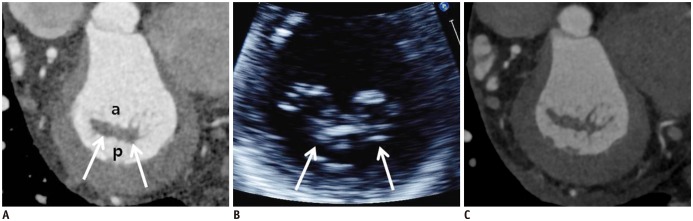
Fig. 1. Normal CT images of MV.
A. Reconstructed short-axis view (en face view) showing each MV leaflet scallop (A1–A3 and P1–P3). In lateral portion of the mitral annulus, LAA and LCx are shown, and in anterior portion, aorta is seen. These landmarks are used for performing reconstruction in en face view of MV. B. Reconstructed three-chamber view (sagittal view) showing anterior leaflet (arrow) and posterior leaflet (dotted arrow) of MV. Fibrous continuity (arrowhead) is identified between aorta and anterior mitral leaflet. C. Reconstructed two-chamber view (coronal view) showing leaflets (arrow), chordae tendineae (dotted arrow), and papillary muscles (asterisks). Posterior leaflet of MV is divided into P1 (lateral), P2 (middle), and P3 (medial) scallop, respectively, opposing segments of anterior leaflet are named A1 (lateral), A2 (middle), and A3 (medial) scallops, respectively. AL = anterolateral commissure, Ao = aorta, CT = computed tomography, LA = left atrium, LAA = left atrial appendage, LCx = left circumflex artery, LV = left ventricle, MV = mitral valve, PM = posteromedial commissure
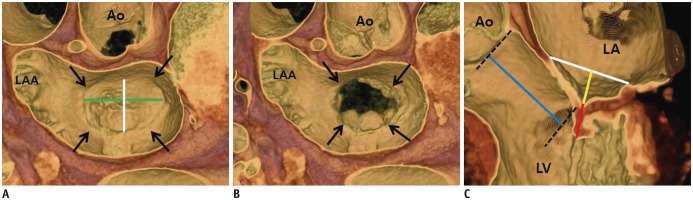
Fig. 2. MV coaptation geometry.
A, B. 3D volume-rendered CT images (en face view) showing mitral annulus (arrows). Intercommissural distance (green line) and septo-lateral distance (white line) can be measured in systolic (A) and diastolic phases (B). C. Geometric parameters such as septo-lateral distance (white line), tenting height (yellow line), coaptation length (red line), and lateral displacement of coaptation (blue line) can be observed and measured in three-chamber view of MV by using 3D volume-rendered CT image. 3D = three-dimensional
Mitral Annulus
The mitral annulus is a junctional zone that separates the left atrium from the left ventricle, and provides support for the MV leaflets. This dynamic structure changes shape during the cardiac cycle. When viewed in three dimensions, it is non-planar, saddle-shaped, and has a commissural diameter of the MV is larger than the anteroposterior diameter (6,8,9,10). The aortic mitral leaflet (anterior) is in fibrous continuity with the aortic valve and the right and left fibrous trigones of the MV (11). Thus, the annulus region is fibrous and less prone to dilation. However, the posterior mitral annulus is largely muscular and more loosely connected to the surrounding tissue, allowing the posterior mitral annulus to move freely during myocardium contraction and relaxation (12). In cases of significant MR, the posterior region often dilates and is more prone to calcification (7).
MV Leaflets
The MV is composed of two leaflets known as the anterior and posterior leaflets; however, respective preferred terms are the aortic and mural leaflets (13). The mural leaflet is narrow, accounting for one-third of the annular circumference around the left atrioventricular junction. Furthermore, the mural leaflet has indentations and forms three scallops. Functional analysis of the MV leaflet by Carpentier classification revealed the following segments: the most lateral segment, P1, which is in continuity with the anterolateral commissure; the central segment, P2, which can vary significantly in size; and the most medial segment, P3, which is in continuity with the posteromedial commissure (14). The MV aortic leaflet accounts for two-thirds of the annular circumference. Based on the number of neighboring mural leaflet regions, the aortic leaflet can be divided into three regions i.e., A1, A2, and A3. The aortic leaflet is also divided into three regions, A1, A2, and A3 based on the number of neighboring mural leaflet regions.
Chordae Tendineae
The heart strings or cord-like tendons known as the chordae tendineae are papillary muscles extending toward and inserted into the MV leaflets. Depending on where they attach, the chordae tendineae may be classified into three types: 1) primary, 2) secondary, and 3) marginal. Primary chords connect to the free edges of both leaflets in the rough zone. Secondary chords connect to the ventricular surface of both leaflets in the rough zone. The largest and thickest chords among the secondary chords of the aortic leaflet are the strut chords. The tertiary chords attach to the junction between the mural leaflet and the ventricular wall (7,15,16).
Papillary Muscles
The papillary muscles located along the mid to apical segments of the left ventricle are usually called the anterolateral and posteromedial muscles. The papillary muscles are labeled this way based on their respective projected relationships to the mitral commissures (17).
MV Coaptation Geometry
Imaging enables the morphological and functional assessments of the MV apparatus. For instance, measuring the MV annulus and leaflet geometry is possible by using various 3D or 4D-imaging reconstruction methods for cardiac CT. The anteroposterior (septo-lateral distance) and inter-commissural diameters can be measured at the level of the MV annulus. The tenting height, coaptation length, and lateral displacement of coaptation can be measured at the mitral coaptation level with clear visualization of both commissures (Fig. 2) (3,7,18,19,20,21).
Carpentier Surgical Classification of MV Pathology
Mitral regurgitation can be classified into three main pathological types based on leaflet motion: normal leaflet motion (type I), leaflet prolapsed or excessive leaflet motion (type II), and restricted leaflet motion (type III). Type III is subdivided into types IIIa (leaflet restriction during the diastole and systole phases) and IIIb (leaflet restriction during the systole phase) (Table 1) (22).
Table 1. Carpentier Surgical Classification of Pathology of Mitral Valve.
| Dysfunction | Lesions | Lesions |
|---|---|---|
| Type I | Annular dilatation Leaflet perforation/tear |
Dilated cardiomyopathy Endocarditis |
| Type II Excess leaflet motion (leaflet prolapse) |
Elongation/ruptured chordae Elongation/rupture papillary muscle |
Degenerative valve disease Fibroelastic deficiency Barlow's disease Marfan disease Endocarditis Trauma Ischemic cardiomyopathy |
| Type IIIa Restricted leaflet motion (diastole and systole phases) |
Leaflet thickening/retraction Leaflet calcification Chordal thickening/retraction/fusion Commissural fusion |
Rheumatic heart disease Carcinoid heart disease |
| Type IIIb Restricted leaflet motion (systole phase) |
Left ventricular dilatation/aneurysm Papillary muscle displacement Chordae tethering |
Ischemic/dilated cardiomyopathy |
MV Repair
Mitral valve diseases have been successfully treated for the last four decades by replacing the native MV with prosthetics. However, using a prosthetic MV has some disadvantages. For instance, infective endocarditis, embolization, coagulopathy disorders and cerebral vascular disease may occur. Thus, some surgeons attempt to repair the damaged native MV to avoid MV replacement complications. The MV annuloplasty technique has progressively improved since its initial introduction by Prof. Alain Carpentier in 1969. Currently, it is the gold standard for treating MV dysfunction. Also, MV repair is commonly associated with improved mid-term and long-term survival compared with MV replacement (23,24,25).
Indications
Based on the American College of Cardiology/American Heart Association guidelines (23), MV surgery (class I) is recommended for symptomatic patients with chronic severe primary MR and left ventricular (LV) ejection fraction (LVEF) > 30%, and asymptomatic patients with chronic severe primary MR and LV dysfunction (LVEF 30–60% and/or an LV end-systolic diameter ≥ 40 mm). MV repair, which is preferable to MV replacement, is recommended for the surgical treatment of patients with chronic severe primary MR that is limited to the posterior leaflet and involves the anterior leaflet or both leaflets, and when the pre-operative tests indicate that a successful and durable repair can be achieved.
Surgical Approaches
Artificial Neochordae
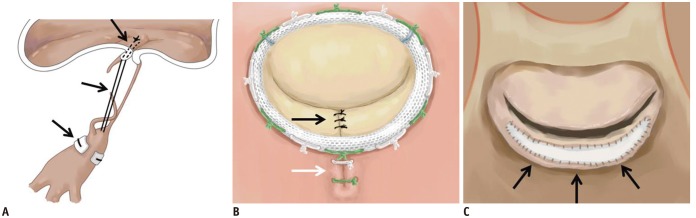
Artificial neochordae can be constructed by coupling the mitral leaflet to the corresponding papillary muscle of damaged or ruptured chordae with the aid of polytetrafluoroethylene sutures (Fig. 3A) (26,27).
Fig. 3. Illustration of MV repair.
A. Artificial neochordae with polytetrafluoroethylene sutures (black arrows). B. Carpentier-Edwards Physio ring with quadrangular resection of posterior mitral leaflet (black arrow) and plication sutures of posterior mitral annulus (white arrow). C. Pericardial patch leaflet augmentation (black arrows).
Quadrangular and Triangular Resection of the Posterior Leaflet
During this procedure, the prolapsed leaflet is excised from the leaflet margin to the annulus; thus, a quadrangular section of the leaflet tissue is removed. Plication sutures are placed along the posterior annulus in the resected area. Subsequently, the resection gap margins are approximated with interrupted polypropylene sutures to restore leaflet continuity (Fig. 3B) (28).
Pericardial Patch Leaflet Augmentation
Diastolic restriction of the leaflet in type IIIa regurgitation cases can be corrected with leaflet augmentation. To perform this procedure, a transverse incision is made 5 mm from the posterior annulus. An elliptical patch is fashioned from the pericardium, and then it is sutured to the incised site to increase the leaflet height (Fig. 3C) (29).
Commissuroplasty
This method of repair involves surgical resection of the prolapsed area and sliding plasty of the adjacent paracommissural segments to reconstruct the prolapsed MV (30).
Mitral Annuloplasty
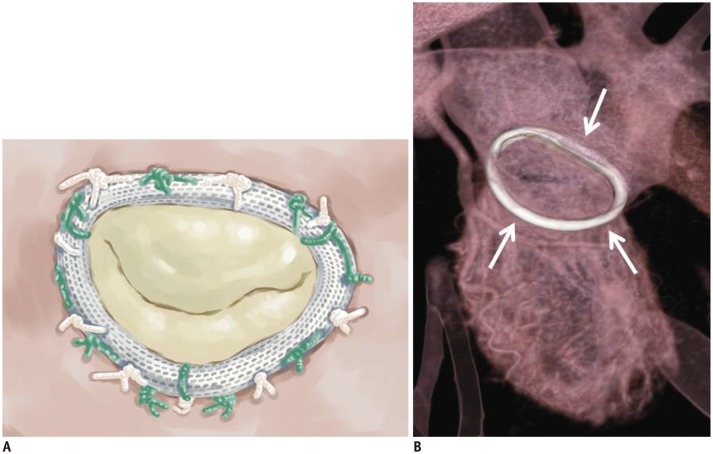
Mitral annuloplasty is a key procedure for MV repair. Annuloplasty rings have many types: complete or incomplete, rigid or flexible, and planar or saddle-shaped. Complete rings (e.g., the Carpentier-Edwards Physio annuloplasty ring) are sutured to the anterior and posterior annuli (Fig. 4), whereas partial rings (e.g., a Cosgrove-Edwards annuloplasty system) are sutured at the right and left trigones and at the posterior annulus, including the anterolateral and posteromedial commissures (Fig. 5). Ring annuloplasty restores the normal physiological size and shape of the annulus, and it improves leaflet coaptation. The procedure also improves the durability of the repair by preventing delayed annular dilation (14,22,31). Posterior mitral annuloplasty strips (Mitra-Lift® Strip, Scien-City, Inc., Seoul, Korea) are flat Dacron strips that are placed only along the posterior annulus and spare the anterior half of both commissures and the anterior annulus. The middle portion of the posterior annulus is lifted because of its curvilinear profile (Fig. 6) (32,33).
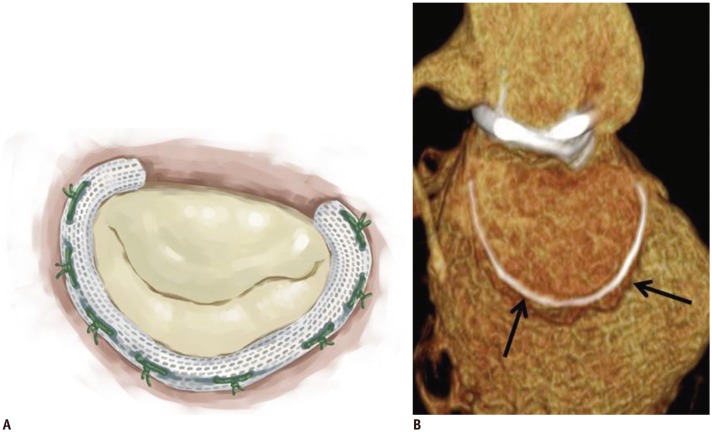
Fig. 4. Carpentier-Edwards Physio annuloplasty ring.
Illustrative image (A) and CT image (B) showing high-density ring structure that surrounds annulus, i.e., Carpentier-Edwards Physio annuloplasty ring (white arrows).
Fig. 5. Cosgrove-Edwards annuloplasty system.
Illustrative image (A) and CT image (B) showing Cosgrove-Edwards annuloplasty system (black arrows) that partially surrounds mitral annulus.
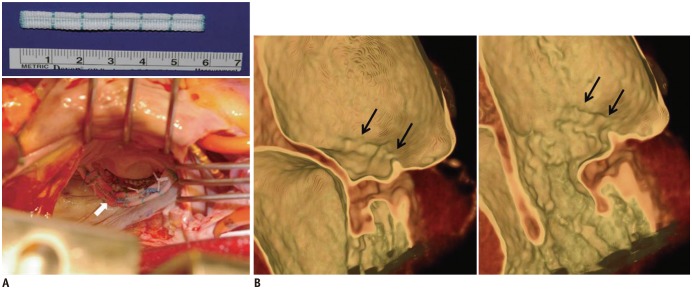
Fig. 6. Intraoperative photograph and post-operative CT images after posterior mitral annuloplasty with ML strip.
A. Intraoperative photograph showing posterior mitral annuloplasty strip (white arrow) placed on atrial wall 5.0 mm above posterior annulus, which decreased posterior annular circumference and spared anterior annulus and both commissures. B. Ridge-like lesion (black arrows) was identified on post-operative CT images during systolic and diastolic phases. ML strip = Mitra-Lift® strip
Pre-Operative and Post-Operative Imaging of the MV
CT Imaging Techniques
At our institution, cardiac CT before MV surgery is mainly performed for MV and coronary artery evaluations using a second-generation dual-source CT scanner (Definition Flash, Siemens, Erlangen, Germany). We used a retrospective electrocardiographically-gated spiral scanning mode and electrocardiography-based current modulation to reconstruct images. A full-strength tube current is used during 30% to 80% of the R-R interval (the interval between two R peaks in the electrocardiogram [the time between two heartbeats]). For this protocol, the peak tube voltage is set as low as possible, at approximately 80–100 kVp depending on the size of the patient's body, to reduce radiation exposure. If the patient's heart rate is higher than 75 beats per minute at 1 hour before the CT examination, 2.5-mg bisoprolol (Conbloc, Elyson Pharmaceutical, Co., Ltd., Seoul, Korea) is sublingually administered. The protocol for contrast material injection is the same protocol used for CT angiography to evaluate the coronary arteries. Depending on the patient's weight, a 70–90 mL bolus of an intravenous non-ionic contrast medium (Xenetix, Guerbet, Roissy, France) is injected at a rate of 4.0–5.0 mL/s rate using a power injector (Stellant D, Medrad, Indianola, PA, USA), followed by a 40-mL saline flush with the bolus-tracking technique (ascending aorta; attenuation threshold, 100 Hounsfield unit; scan delay, 7 seconds). Acquisition parameters are as follows: section thickness, 0.6 to 0.75 mm; tube voltage range, 80 to 120 kV; tube current-time product, 185 to 380 mAs; collimation, 128 × 0.6 mm; and gantry rotation time, 280 ms. After transferring multiphase source data to an external workstation (AquariusNet, TeraRecon, Foster City, CA, USA), post-processing is performed for image reconstruction.
Imaging Reconstruction
After reviewing the multiphase CT data, we selected the best cardiac cycle phase for the MV evaluation. Koo et al. (34) reported that the best quality images were obtained during the phases at 25% to 35% of the cardiac cycles in patients with MV prolapse. To more accurately assess the MV anatomy and geometry using CT, the reconstructed short-axis view should be generated after precisely selecting the mitral annulus plane in four- and two-chamber views. Koo et al. (34) introduced the en face view of the MV, i.e., the surgeon's view. By setting the images on the en face view, either perpendicular or parallel to the coaptation line, the MV sagittal or coronal views, respectively, can be obtained by using a multiplanar reformatting display technique (Fig. 1). Furthermore, methods that render 3D cardiac cine reconstruction of the images may be helpful.
MV CT Imaging
In MV repair, the operative field finding is important in deciding on the surgery type. However, evaluating MV function in the operative field without ventricular dynamic compression has limitations during cardiac arrest. In cardiac surgeries, cardiopulmonary bypass and aortic cross-clamping times are important risk factors for survival and risks of complications (35). Therefore, pre-operative findings of MV structure and dynamic movement are important. Pre-operative CT can provide the structural details, such as calcification, valvular thickening, subvalvular structure, such as papillary muscle and ventricular cavity, and annular stenosis with fibrosis. Blood flow can be evaluated more precisely in echocardiography. However, the evaluation of the entire mitral valvular and subvalvular structures and dynamic movement has a limitation in echocardiography. Therefore, cardiac CT can be an additional important evaluation tool in deciding for the best surgical method (plasty or replacement).
Multidetector row CT enables accurate diagnosis of MV prolapse (Fig. 7) (34,36,37). Also, Delgado et al. (3) demonstrated that multislice CT allows a comprehensive assessment of the MV apparatus by giving an exact characterization of subvalvular apparatus and MV geometry. This study showed that patients with heart failure with moderate to severe functional MR had a more pronounced tethering at the central and posteromedial levels of mitral leaflets. These CT findings can provide important inferences for MV repair in patients with heart failure with severe functional MR.
Fig. 7. 52-year-old man who presented with chest discomfort.
Pre-operative CT images of MV. MV (arrows) was reconstructed by using multiplanar reformat display technique and 3D volume-rendered image with thin-slap reformation technique. En face view (A) and three-chamber view of 3D volume-rendered image (B) at level of MV during systolic phase showing prolapse at A2–A3 level of anterior mitral leaflet. Color Doppler echocardiography showed moderate degree of eccentric mitral regurgitation.
Recent advances in 3D imaging techniques have allowed for a better understanding of pre-operative and post-operative changes in the MV anatomy and geometry (8,9,18,38). By using 3D echocardiography, Mahmood et al. (18) showed that the MV geometry might vary depending on the nature of the implanted prosthetic device. Although follow-up imaging after MV repair is primarily achieved in routine clinical practice by using echocardiography, CT is preferred for post-operative evaluations since the spatial resolution is better and susceptibility to metallic artifacts is decreased (Figs. 8, 9, 10, 11) (Supplementary Movie 1, 2, 3, 4, 5 in the online-only Data Supplement) (1,39). However, cardiac CT for MV evaluation has several disadvantages (Table 2) (40,41). CT has a latent risk from ionizing radiation (38). Also, radiation doses will be increased to obtain a systolic phase to assess the MV (34). The inferior temporal resolution requires medication to control high or irregular heartbeats compared with echocardiography (41). Because iodinated contrast material injection is required to evaluate the cardiac structures, including cardiac valves, cardiac CT is contraindicated in some patients. Furthermore, hemodynamic information through the MV cannot be evaluated in cardiac CT (38,42). Despite these disadvantages, evaluation of the LV geometry in cases of aortic valve disease with a functional MR by using CT is helpful when planning a surgery, and changes in the geometry of both the aortic valve and MV can be clearly identified post-operatively (Fig. 10) (Supplementary Movie 3, 4 in the online-only Data Supplement) (38,43,44). By using 3D-reconstructed CT images, Kim et al. (32) demonstrated that posterior annuloplasty with a novel strip resulted in a sufficient coaptation height, secondary to a decrease in the anteroposterior annular diameter and the annular geometric changes after MV repair (Figs. 6, 11) (Supplementary Movie 5 in the online-only Data Supplement). The normal MV annulus is a non-planar, saddle-shaped structure (6,8,9,10). Thus, the non-planarity of the annulus is considered to contribute to reducing leaflet stress (45,46). The goals of MV repair are to preserve or restore full leaflet motion, create a large surface of coaptation, and remodel and stabilize the entire annulus (22). Therefore, to restore non-planarity after repair, the introduction of specific surgical repair techniques and prostheses is warranted (47). Consequently, the detailed assessment of the MV geometry before and after MV repair by using cardiac CT may help surgeons select better options that consequently facilitate a good clinical outcome.
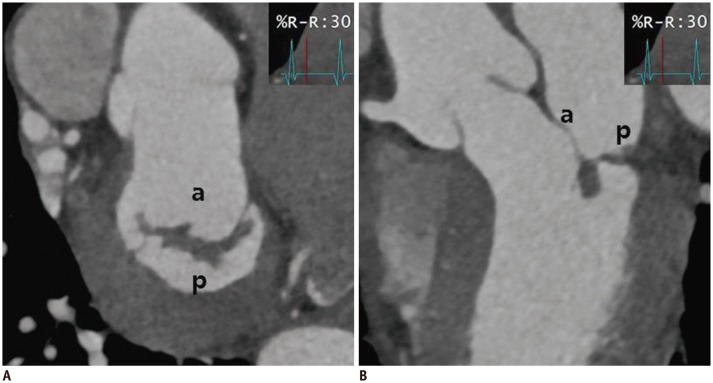
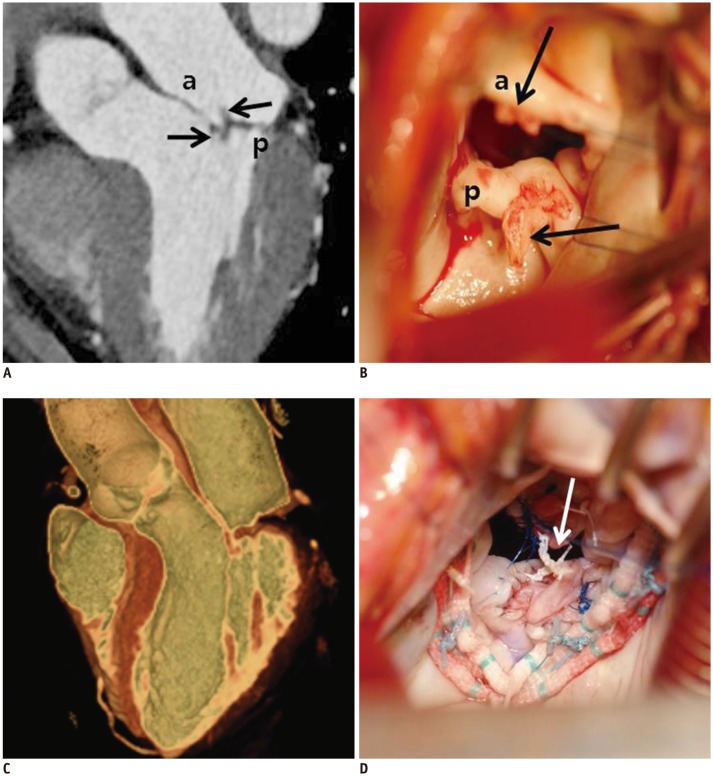
Fig. 8. Cardiac CT images after MV repair due to mitral regurgitation caused by MV prolapse.
En face view (A) and three-chamber view (B) at level of mitral valve during systolic phase showing well functioning mitral valve without coaptation defect. a = anterior leaflet of MV, p = posterior leaflet of MV
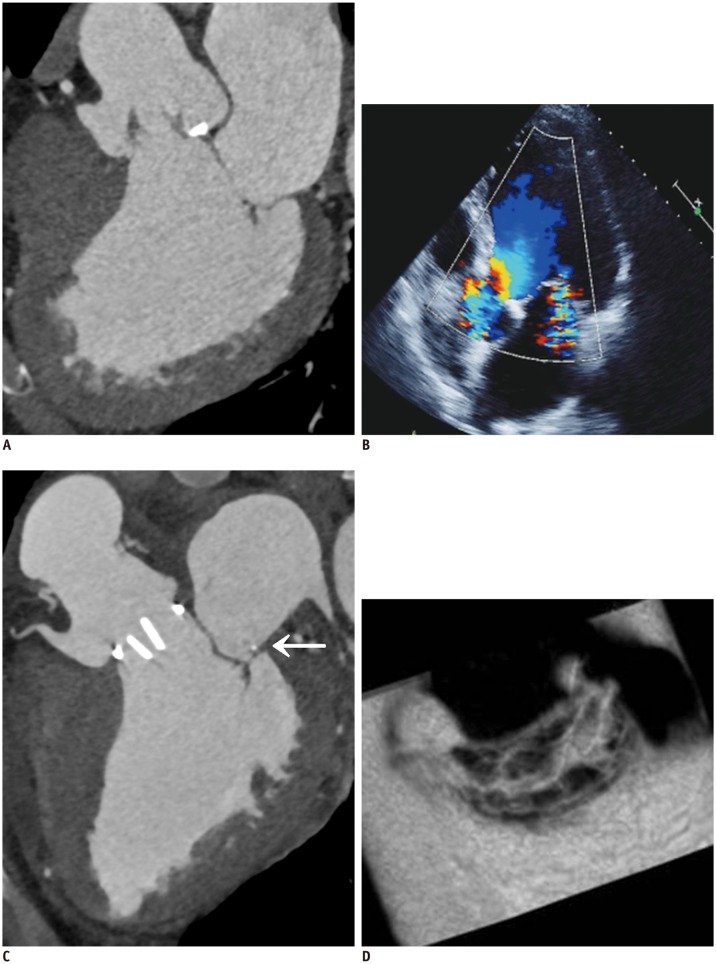
Fig. 9. Pre-operative and post-operative cardiac CT and echocardiographic images of 59-year-old man who presented with palpitation.
A. En face views at level of MV (white arrows) during systolic phase showing diffuse valvular thickening without definite mitral stenosis or regurgitation. Coaptation failure was not likely according to multiplanar reformatted images. B. Diffuse valvular thickening (white arrows) was also shown on echocardiography. Color Doppler echocardiography showed moderate degree of mitral regurgitation. C. Diffuse valvular thickening decreased after MV repair, but post-operative cardiac CT still showed small coaptation defect in MV.
Fig. 10. 63-year-old man who presented with exertional dyspnea.
A. Pre-operative cardiac CT of multiplanar reformatted images in three-chamber view of MV. B. Echocardiographic image is showing functional mitral regurgitation combined with bicuspid aortic valve with eccentric AR and aneurysmal dilatation of ascending aorta. C, D. Aortic valve replacement and ascending aortic aneurysm resection with graft interposition and Cosgrove-Edwards annuloplasty (C, arrow) were performed for functional mitral regurgitation. Post-operative cardiac CT of multiplanar reformatted images in three-chamber view (C) and en face view of MV (D). En face view of MV show well-functioning MV with good coaptation (D). AR = atrial regurgitation
Fig. 11. 57-year-old woman who presented with necrosis of left toe and finger.
Pre-operative cardiac CT was obtained to evaluate MV and rule out infective endocarditis with vegetation.
A. Three-chamber view at level of MV was reconstructed. There were two small soft tissue density masses (black arrows) in anterior and posterior leaflets of MV with mild coaptation defect. B. Intraoperative photograph demonstrating two small masses (black arrows) in anterior and posterior leaflets of MV with endocarditis. Pre-operative echocardiography showed thickened MV with shaggy echogenic material on anterior and posterior mitral leaflets and mild mitral regurgitation. C. Cardiac CT after MV repair. MV was reconstructed by using 3D volume-rendered image with thin-slap reformation technique; well-functioning MV without coaptation defect was observed. D. Two small masses were removed with entire layers of leaflet. Leaflet defects were repaired with autologous pericardial patches and posterior mitral annuloplasty. Artificial neochordae (arrow) were replaced. Histological diagnosis of two small masses was confirmed as papillary fibroelastoma.
Table 2. Advantages and Disadvantages of MV Imaging between Echocardiography and Cardiac CT.
| Echocardiography | Cardiac CT | |
|---|---|---|
| Advantages | Fundamental information: valve disease including flow measurements Readily available, cost-effective and safe |
High spatial resolution Detailed depiction of MV anatomy Simultaneous evaluation of adjacent anatomic structures Rapid acquisition Collection of three-dimensional dataset Wide availability during off-hours |
| Disadvantages | Operator-dependence High interobserver variability Poor acoustic windows |
Radiation exposure Need for iodinated contrast material Inferior temporal resolution No hemodynamic information |
MV = mitral valve
CONCLUSION
Cardiac CT enables clinicians to perform a detailed assessment of the MV anatomy and geometry before and after surgery, regardless of the surgical method. Therefore, cardiac CT can be used as an alternative imaging modality to echocardiography for evaluating the causes of MV disease pre-operatively and predicting the prognosis with the MV geometry measurement after MV repair.
Acknowledgments
The authors thank Dr. Dong Hyun Yang for his valuable assistance. This paper was supported by the Fund of Biomedical Research Institute, Chonbuk National University Hospital, Korea.
Supplementary Movie Legends
This video shows CT images of well-functioning mitral valve (MV) after MV repair using a 3D volume-rendered cine image. The post-operative change in the annular and leaflet geometry is well demonstrated in this video.
This video shows CT images obtained from the en face view of mitral valve (MV) after MV repair (posterior annuloplasty with ML strip) in a patient with rheumatic mitral stenosis and regurgitation. The reduction in thickening and coaptation defect of the MV after MV repair is better demonstrated in this video than was shown in Figure 9C.
This video shows CT images of well-functioning mechanical aortic valve and mitral valve at the same time after aortic valve replacement and Cosgrove-Edwards annuloplasty in a patient with bicuspid aortic valve and functional mitral regurgitation.
It is difficult to accurately determine whether the coaptation defect remained in the multiplanar reformatted images of the cardiac CT (Figure 10C), but it is evident in the 3D volume-rendered cine image obtained from a en face view of the mitral valve.
This video shows well-functioning mitral valve (MV) without coaptation defect after MV repair (posterior annuloplasty with ML strip) as shown in Figure 11C. Using a 3D volume-rendered cine image, the artificial neochordae are clearly visible and the relationship between the MV and the surrounding structures is well demonstrated.
References
- 1.Chen JJ, Manning MA, Frazier AA, Jeudy J, White CS. CT angiography of the cardiac valves: normal, diseased, and postoperative appearances. Radiographics. 2009;29:1393–1412. doi: 10.1148/rg.295095002. [DOI] [PubMed] [Google Scholar]
- 2.Shanks M, Delgado V, Ng AC, van der Kley F, Schuijf JD, Boersma E, et al. Mitral valve morphology assessment: three-dimensional transesophageal echocardiography versus computed tomography. Ann Thorac Surg. 2010;90:1922–1929. doi: 10.1016/j.athoracsur.2010.06.116. [DOI] [PubMed] [Google Scholar]
- 3.Delgado V, Tops LF, Schuijf JD, de Roos A, Brugada J, Schalij MJ, et al. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc Imaging. 2009;2:556–565. doi: 10.1016/j.jcmg.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ, Yong HS, Kim SM, Kim JA, Yang DH, Hong YJ, et al. Korean guidelines for the appropriate use of cardiac CT. Korean J Radiol. 2015;16:251–285. doi: 10.3348/kjr.2015.16.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dal-Bianco JP, Levine RA. Anatomy of the mitral valve apparatus: role of 2D and 3D echocardiography. Cardiol Clin. 2013;31:151–164. doi: 10.1016/j.ccl.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, et al. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80:589–598. doi: 10.1161/01.cir.80.3.589. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy KP, Ring L, Rana BS. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr. 2010;11:i3–i9. doi: 10.1093/ejechocard/jeq153. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan SR, Bashein G, Sheehan FH, Legget ME, Munt B, Li XN, et al. Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am Heart J. 2000;139:378–387. doi: 10.1016/s0002-8703(00)90077-2. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe N, Ogasawara Y, Yamaura Y, Wada N, Kawamoto T, Toyota E, et al. Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation. 2005;112(9 Suppl):I458–I462. doi: 10.1161/CIRCULATIONAHA.104.524595. [DOI] [PubMed] [Google Scholar]
- 10.Fedak PW, McCarthy PM, Bonow RO. Evolving concepts and technologies in mitral valve repair. Circulation. 2008;117:963–974. doi: 10.1161/CIRCULATIONAHA.107.702035. [DOI] [PubMed] [Google Scholar]
- 11.Ormiston JA, Shah PM, Tei C, Wong M. Size and motion of the mitral valve annulus in man. I. A two-dimensional echocardiographic method and findings in normal subjects. Circulation. 1981;64:113–120. doi: 10.1161/01.cir.64.1.113. [DOI] [PubMed] [Google Scholar]
- 12.Puff A. Ischemic mitral incompetence. New York: Springer; 1991. [Google Scholar]
- 13.Angelini A, Ho SY, Thiene G, Anderson RH. Anatomy of the mitral valve. In: Boudoulas H, Wooley CF, editors. Mitral valve: floppy mitral valve, mitral valve prolapse, mitral valvular regurgitation. 2nd ed. New York: Futura Publishing Company; 2000. pp. 5–29. [Google Scholar]
- 14.Carpentier AF, Lessana A, Relland JY, Belli E, Mihaileanu S, Berrebi AJ, et al. The “physio-ring”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg. 1995;60:1177–1185. doi: 10.1016/0003-4975(95)00753-8. discussion 1185-1186. [DOI] [PubMed] [Google Scholar]
- 15.Lam JH, Ranganathan N, Wigle ED, Silver MD. Morphology of the human mitral valve. I. Chordae tendineae: a new classification. Circulation. 1970;41:449–458. doi: 10.1161/01.cir.41.3.449. [DOI] [PubMed] [Google Scholar]
- 16.Degandt AA, Weber PA, Saber HA, Duran CM. Mitral valve basal chordae: comparative anatomy and terminology. Ann Thorac Surg. 2007;84:1250–1255. doi: 10.1016/j.athoracsur.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Rusted IE, Scheifley CH, Edwards JE. Studies of the mitral valve. I. Anatomic features of the normal mitral valve and associated structures. Circulation. 1952;6:825–831. doi: 10.1161/01.cir.6.6.825. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood F, Subramaniam B, Gorman JH, 3rd, Levine RM, Gorman RC, Maslow A, et al. Three-dimensional echocardiographic assessment of changes in mitral valve geometry after valve repair. Ann Thorac Surg. 2009;88:1838–1844. doi: 10.1016/j.athoracsur.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muresian H. The clinical anatomy of the mitral valve. Clin Anat. 2009;22:85–98. doi: 10.1002/ca.20692. [DOI] [PubMed] [Google Scholar]
- 20.Noack T, Kiefer P, Ionasec R, Voigt I, Mansi T, Vollroth M, et al. New concepts for mitral valve imaging. Ann Cardiothorac Surg. 2013;2:787–795. doi: 10.3978/j.issn.2225-319X.2013.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen H, Jensen MO, Smerup MH, Vind-Kezunovic S, Ringgaard S, Andersen NT, et al. Impact of papillary muscle relocation as adjunct procedure to mitral ring annuloplasty in functional ischemic mitral regurgitation. Circulation. 2009;120(11 Suppl):S92–S98. doi: 10.1161/CIRCULATIONAHA.108.817833. [DOI] [PubMed] [Google Scholar]
- 22.Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–337. [PubMed] [Google Scholar]
- 23.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148:e1–e132. doi: 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YX, Leobon B, Berthoumieu P, Roux D, Glock Y, Mei YQ, et al. Long-term outcomes following repair or replacement in degenerative mitral valve disease. Thorac Cardiovasc Surg. 2010;58:415–421. doi: 10.1055/s-0029-1240925. [DOI] [PubMed] [Google Scholar]
- 25.Goldstone AB, Woo YJ. Surgical treatment of the mitral valve. In: Sellke FW, del Nido PJ, Swanson SJ, editors. Sabiston and Spencer surgery of the chest. 9th ed. Philadelphia: Saunders Elsevier; 2016. pp. 1384–1429. [Google Scholar]
- 26.von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg. 2000;70:2166–2168. doi: 10.1016/s0003-4975(00)02047-6. [DOI] [PubMed] [Google Scholar]
- 27.David TE, Omran A, Armstrong S, Sun Z, Ivanov J. Long-term results of mitral valve repair for myxomatous disease with and without chordal replacement with expanded polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg. 1998;115:1279–1285. doi: 10.1016/S0022-5223(98)70210-7. [DOI] [PubMed] [Google Scholar]
- 28.Braunberger E, Deloche A, Berrebi A, Abdallah F, Celestin JA, Meimoun P, et al. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104(12 Suppl 1):I8–I11. [PubMed] [Google Scholar]
- 29.Zegdi R, Khabbaz Z, Chauvaud S, Latremouille C, Fabiani JN, Deloche A. Posterior leaflet extension with an autologous pericardial patch in rheumatic mitral insufficiency. Ann Thorac Surg. 2007;84:1043–1044. doi: 10.1016/j.athoracsur.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 30.van Herwerden LA, Taams MA, Bos E. Repair of commissural prolapse by extended leaflet sliding. Ann Thorac Surg. 1994;57:387–390. doi: 10.1016/0003-4975(94)91002-2. [DOI] [PubMed] [Google Scholar]
- 31.Cosgrove DM, 3rd, Arcidi JM, Rodriguez L, Stewart WJ, Powell K, Thomas JD. Initial experience with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg. 1995;60:499–503. doi: 10.1016/0003-4975(95)00458-W. discussion 503-504. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Kim KH, Choi JB, Kuh JH. Posterior mitral annuloplasty for enhancing mitral leaflet coaptation: using a strip designed for placement in the posterior annulus. J Cardiothorac Surg. 2015;10:164. doi: 10.1186/s13019-015-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MG, Shin JK, Chee HK, Kim JS, Yang HS, Choi JB. Lifting posterior mitral annuloplasty for enhancing leaflet coaptation in mitral valve repair: midterm outcomes. Ann Cardiothorac Surg. 2015;4:249–256. doi: 10.3978/j.issn.2225-319X.2015.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo HJ, Yang DH, Oh SY, Kang JW, Kim DH, Song JK, et al. Demonstration of mitral valve prolapse with CT for planning of mitral valve repair. Radiographics. 2014;34:1537–1552. doi: 10.1148/rg.346130146. [DOI] [PubMed] [Google Scholar]
- 35.Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:814–822. doi: 10.1053/j.jvca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Feuchtner GM, Alkadhi H, Karlo C, Sarwar A, Meier A, Dichtl W, et al. Cardiac CT angiography for the diagnosis of mitral valve prolapse: comparison with echocardiography. Radiology. 2010;254:374–383. doi: 10.1148/radiol.2541090393. [DOI] [PubMed] [Google Scholar]
- 37.Shah RG, Novaro GM, Blandon RJ, Wilkinson L, Asher CR, Kirsch J. Mitral valve prolapse: evaluation with ECG-gated cardiac CT angiography. AJR Am J Roentgenol. 2010;194:579–584. doi: 10.2214/AJR.09.2545. [DOI] [PubMed] [Google Scholar]
- 38.Beaudoin J, Thai WE, Wai B, Handschumacher MD, Levine RA, Truong QA. Assessment of mitral valve adaptation with gated cardiac computed tomography: validation with three-dimensional echocardiography and mechanistic insight to functional mitral regurgitation. Circ Cardiovasc Imaging. 2013;6:784–789. doi: 10.1161/CIRCIMAGING.113.000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris MF, Maleszewski JJ, Suri RM, Burkhart HM, Foley TA, Bonnichsen CR, et al. CT and MR imaging of the mitral valve: radiologic-pathologic correlation. Radiographics. 2010;30:1603–1620. doi: 10.1148/rg.306105518. [DOI] [PubMed] [Google Scholar]
- 40.Chheda SV, Srichai MB, Donnino R, Kim DC, Lim RP, Jacobs JE. Evaluation of the mitral and aortic valves with cardiac CT angiography. J Thorac Imaging. 2010;25:76–85. doi: 10.1097/RTI.0b013e31819d12b1. [DOI] [PubMed] [Google Scholar]
- 41.Pham N, Zaitoun H, Mohammed TL, DeLaPena-Almaguer E, Martinez F, Novaro GM, et al. Complications of aortic valve surgery: manifestations at CT and MR imaging. Radiographics. 2012;32:1873–1892. doi: 10.1148/rg.327115735. [DOI] [PubMed] [Google Scholar]
- 42.Guo YK, Yang ZG, Ning G, Rao L, Dong L, Pen Y, et al. Isolated mitral regurgitation: quantitative assessment with 64-section multidetector CT--comparison with MR imaging and echocardiography. Radiology. 2009;252:369–376. doi: 10.1148/radiol.2522081714. [DOI] [PubMed] [Google Scholar]
- 43.Suh YJ, Kim YJ, Hong YJ, Lee HJ, Hur J, Im DJ, et al. Measurement of opening and closing angles of aortic valve prostheses in vivo using dual-source computed tomography: comparison with those of manufacturers’ in 10 different types. Korean J Radiol. 2015;16:1012–1023. doi: 10.3348/kjr.2015.16.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halpern EJ. Clinical applications of cardiac CT angiography. Insights Imaging. 2010;1:205–222. doi: 10.1007/s13244-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salgo IS, Gorman JH, 3rd, Gorman RC, Jackson BM, Bowen FW, Plappert T, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711–717. doi: 10.1161/01.cir.0000025426.39426.83. [DOI] [PubMed] [Google Scholar]
- 46.Bouma W, Aoki C, Vergnat M, Pouch AM, Sprinkle SR, Gillespie MJ, et al. Saddle-shaped annuloplasty improves leaflet coaptation in repair for ischemic mitral regurgitation. Ann Thorac Surg. 2015;100:1360–1366. doi: 10.1016/j.athoracsur.2015.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillinov AM, Cosgrove DM, 3rd, Shiota T, Qin J, Tsujino H, Stewart WJ, et al. Cosgrove-Edwards Annuloplasty System: midterm results. Ann Thorac Surg. 2000;69:717–721. doi: 10.1016/s0003-4975(99)01543-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video shows CT images of well-functioning mitral valve (MV) after MV repair using a 3D volume-rendered cine image. The post-operative change in the annular and leaflet geometry is well demonstrated in this video.
This video shows CT images obtained from the en face view of mitral valve (MV) after MV repair (posterior annuloplasty with ML strip) in a patient with rheumatic mitral stenosis and regurgitation. The reduction in thickening and coaptation defect of the MV after MV repair is better demonstrated in this video than was shown in Figure 9C.
This video shows CT images of well-functioning mechanical aortic valve and mitral valve at the same time after aortic valve replacement and Cosgrove-Edwards annuloplasty in a patient with bicuspid aortic valve and functional mitral regurgitation.
It is difficult to accurately determine whether the coaptation defect remained in the multiplanar reformatted images of the cardiac CT (Figure 10C), but it is evident in the 3D volume-rendered cine image obtained from a en face view of the mitral valve.
This video shows well-functioning mitral valve (MV) without coaptation defect after MV repair (posterior annuloplasty with ML strip) as shown in Figure 11C. Using a 3D volume-rendered cine image, the artificial neochordae are clearly visible and the relationship between the MV and the surrounding structures is well demonstrated.