Figure 7.
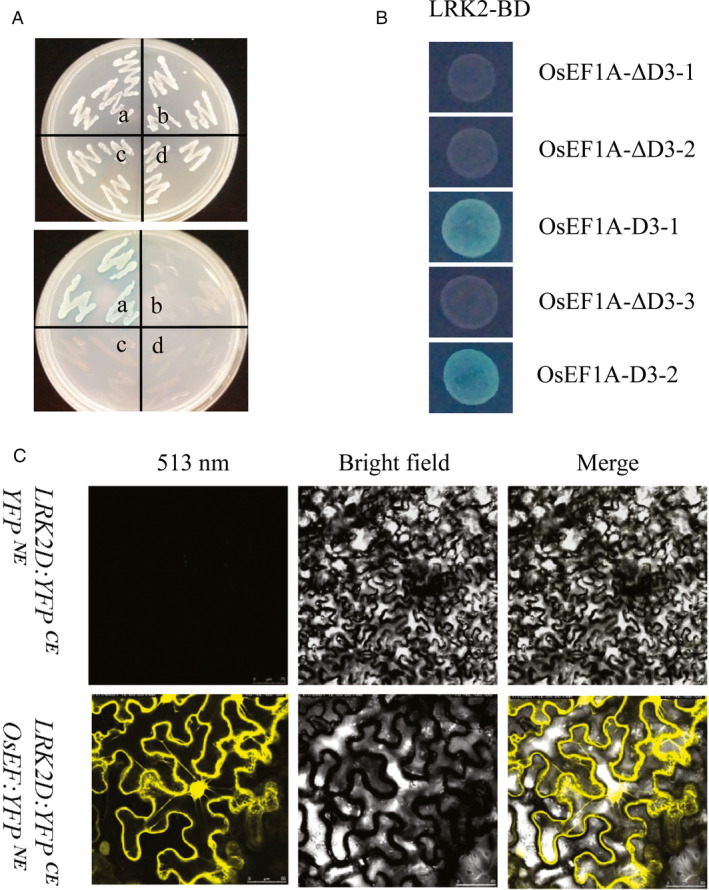
Interaction between LRK2D and OsEF1A in vitro. (A) Interaction between OsEF1A and the LRK2 kinase domain as bait in a yeast two‐hybrid system. Photograph shows the growth behaviour of transformants on SD/Leu‐Trp medium (upper) and SD/Leu‐Trp‐His‐/50 mM3‐AT medium (lower). The yeast cells harboured various pairs of plasmids: a. pGBKT7‐LRK2D with pGADT7‐OsEF1A; b. pGBKT7 with pGADT7‐OsEF1A; c. pGBKT7‐LRK2D with pGADT7; d. pGBKT7 with pGADT7. (B) The carboxyl domain of OsEF1A was found sufficient, and necessary, for interaction with the LRK2 kinase domain in yeast. Residues of OsEF1A present in the constructs were 1 to 230aa (OsEF1A‐ΔD3‐1), 231 to 320aa (OsEF1A‐ΔD3‐2), 321 to 447aa (OsEF1A‐D3‐1), 1 to 320aa (OsEF1A‐ΔD3‐3), 231 to 447aa (OsEF1A‐D3‐2). (C) Interaction between LRK2D and OsEF1A as examined by BiFC. The indicated constructs were transformed into leaves of Nicotiana benthamiana. Scale bars in upper images: 20 mm. Scale bars in lower images: 50 mm.
