Figure 1.
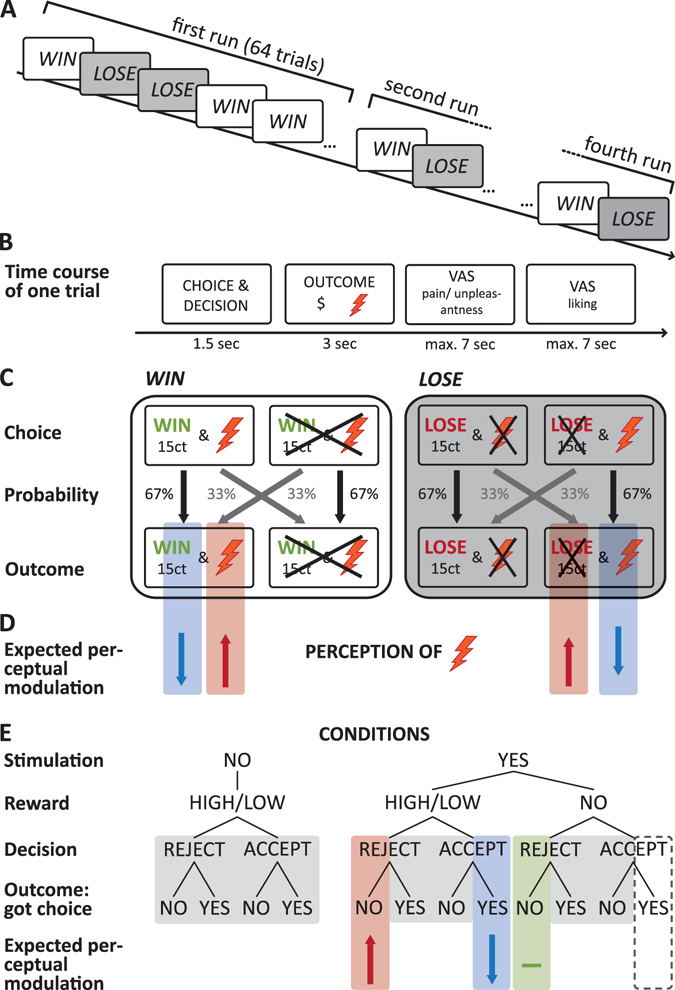
The decision-making task. (A) In four runs of 64 trials each, participants performed win and lose trials in pseudo-randomized order. (B) Time course of one trial: At the beginning of each trial the possible choices were displayed and participants indicated their decision, followed by the presentation of the outcome. If an electrocutaneous stimulus was applied, participants rated how painful and/or unpleasant they perceived this stimulus on a VAS. Subsequently, participants rated in each trials how much they liked the monetary outcome of the trials on a VAS. After a short break (1 sec) the next trial started. (C) In the win trials, participants had to decide whether they wanted to receive a monetary win at the cost of simultaneously receiving a nociceptive stimulus or to reject the win at the benefit of avoiding the pain. In the lose trials, participants had to choose between a monetary loss at the benefit of avoiding a nociceptive stimulus or rejecting the loss at the cost of receiving the stimulus. In both trial types, participants received their choice in 67% of the trials (outcome). (D) Decreased perceived intensity (blue shading) was expected if participants accepted the monetary win coupled to the nociceptive stimulus or when they rejected the loss coupled to the nociceptive stimulus. Increased perceived intensity (red shading) was expected if participants rejected the monetary win to avoid the nociceptive stimulus or when they accepted the loss to avoid the stimulus. (E) Coding of conditions for the statistical analyses: Trials with and without electrocutaneous stimulation could be coupled to low or high reward (in terms of receiving a monetary win or avoiding a monetary loss). Trials with stimulation could be also coupled to no (zero) reward. Participants could accept or reject the reward coupled to the electrocutaneous stimulation and they could get (67%) or not get (33%) their choice. Shaded areas indicate trials with no stimulation. Only for trials with stimulation effects on perceived pain could be analysed. Blue shading indicates the condition for which decreased perceived intensity was expected, red shading increased perceived intensity. Green shading indicates the control condition. The condition highlighted by a dotted line, indicates trials that were excluded from the main analyses because of low occurrences.
