Figure 4.
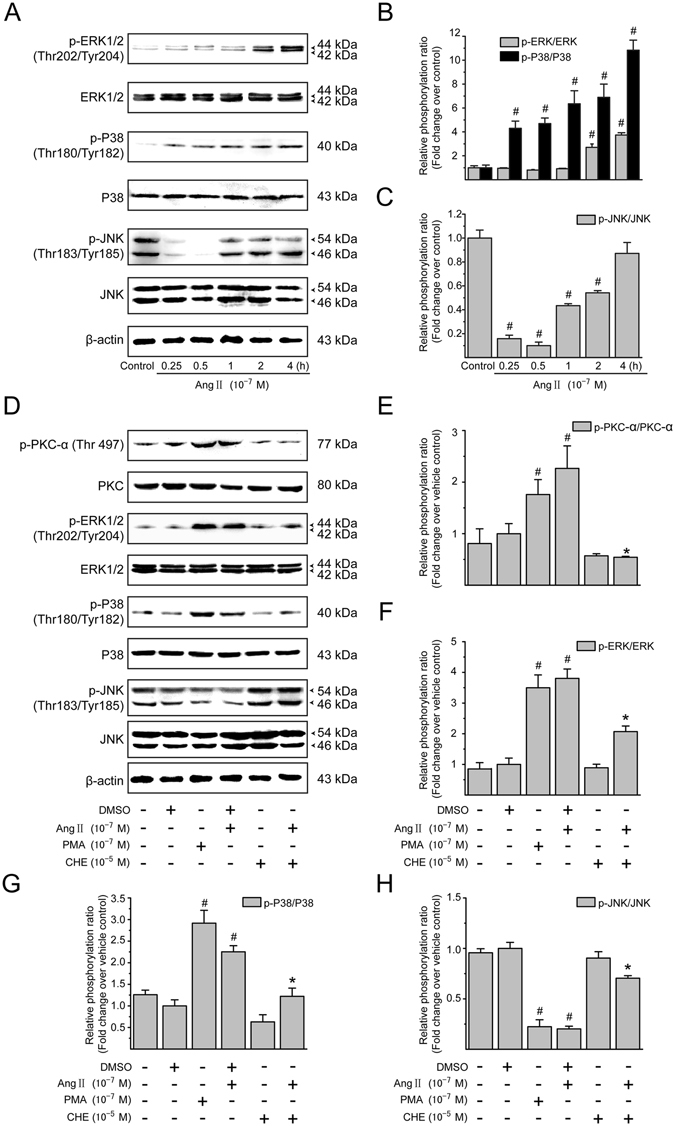
Ang II-mediated MAPK pathways depend on PKC activation in the LX-2 cells. (A) Serum-starved LX-2 cells were incubated with Ang II (10−7 M) for 0, 0.25, 0.5, 1, 2 and 4 h. Whole cell lysates were immunoblotted with antibodies against ERK1/2, p38 MAPK, JNK, and their phosphorylated forms, respectively. Total protein levels of ERK1/2, p38 MAPK and JNK were used as internal controls. Similar results were observed in 3 independent experiments, and representative immunoblot bands are shown. (B,C) Fold-changes in the relative levels of phosphorylated ERK1/2, p38 MAPK and JNK protein are shown after normalizing with the corresponding loading control. The relative protein level in vehicle-treated cells was set as 1.0. (D) Serum-starved LX-2 cells were pretreated with CHE (a PKC inhibitor; 10−5 M) for 0.5 h, and then incubated with or without Ang II (10−7 M) for either 0.5 h (to detect phosphorylated PKC-α, p38 MAPK and JNK) or 2 h (to detect phosphorylated ERK1/2), followed by Western blotting with the indicated antibodies. PMA (10−7 M) was included as a positive control. Total protein levels of PKC-α, ERK1/2, p38 MAPK and JNK were used as loading controls. Similar results were observed in 3 independent experiments, and representative immunoblots for each protein are shown. (E–H) The protein levels of phosphorylated forms of PKC-α, ERK1/2, p38 MAPK and JNK were normalized with the value of respective loading control. It was designated as relative phosphorylation ratio compared with that in vehicle-treated cells (defined as 1-fold). Results are presented as mean ± SD of 3 independent experiments. # P < 0.05 versus unstimulated or vehicle-treated control cells; * P < 0.05 versus Ang II-treated cells.
