Figure 1.
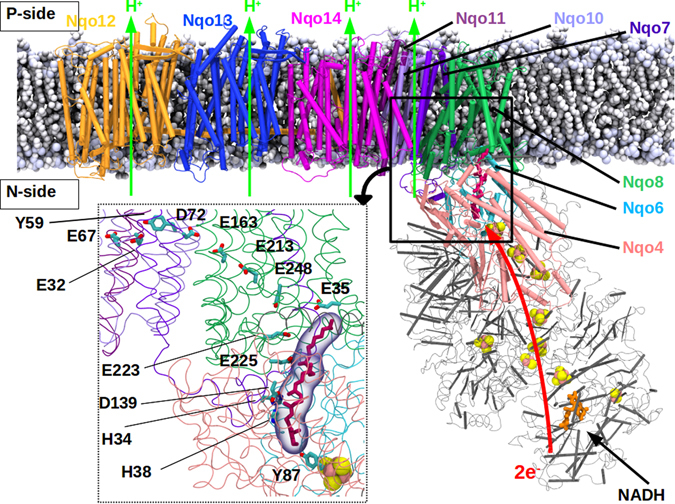
Structure of respiratory complex I embedded in lipid-solvent environment. The membrane-embedded antiporter-type subunits Nqo12–14 are shown in orange, blue and magenta, respectively. Nqo8 (green), Nqo7, Nqo10 and Nqo11 (violet-indigo) are also displayed along with the hydrophilic subunits Nqo6 (light blue) and Nqo4 (pink) that form the Q-binding site scaffold. Q (purple) binds in a buried binding site. All other hydrophilic subunits are shown in grey. Covalently-bound FeS clusters (pink-yellow spheres), and FMN (orange) at the terminal end of the hydrophilic domain are also shown. NADH binds next to FMN and acts as a direct donor of two electrons to the latter. The region studied in this work is marked by a thick black box, and is shown to atomic details in the inset (box with dotted lines). The residues discussed in this work are also marked (inset).
