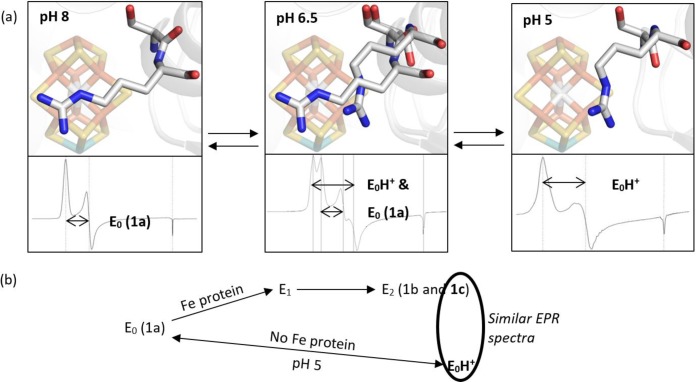
Figure 5.
(a) A summary of the Cp1 data presented in this manuscript. At pH 8, the typical resting state X-ray diffraction structure and EPR signal are observed. At pH 5, a peptide flip and repositioning of the α-Arg357 side chain away from the Fe3,4,5,7 face of the FeMo-cofactor is observed as well as a S = 3/2 spin system with zero-field splitting parameters similar to those reported for one of the signals observed in the E2 state. At intermediate pH, both structural conformations and EPR spin systems are observed. The EPR signals and X-ray structures are reversible and correlated. (b) The 1c peak has been attributed to the E2 state and is hypothesized to result from protonation of the FeMo-cofactor. Our experimental conditions include only a proton source and not an electron source, so it is unlikely that these conditions achieve a reduced state, such as E2. Consequently, we propose that our low pH conditions yield a protonated resting state, which we call “E0H+”.