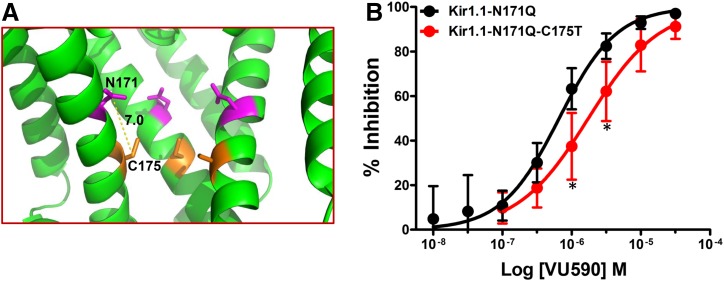
Fig. 7.
Substitution of a threonine residue at position 175 reduces block of a binding-site mutant Kir1.1 channel by VU590. (A) Magnified pore lining section of the Kir1.1 homology model from Fig. 1A showing relative distance between N171 (magenta) and T175 (orange) residues. (B) CRC showing dose-dependent inhibition of Kir1.1-N171Q or Kir1.1-N171Q-C175T channels. The C175T reverse mutation shows a rightward shift in the CRC and is statistically significantly different at 1 and 3 μM concentrations. Data were analyzed using one-way ANOVA with Bonferroni’s multiple comparison test. *P < 0.05, statistically significantly different from Kir1.1-N171Q, n ≥ 6.