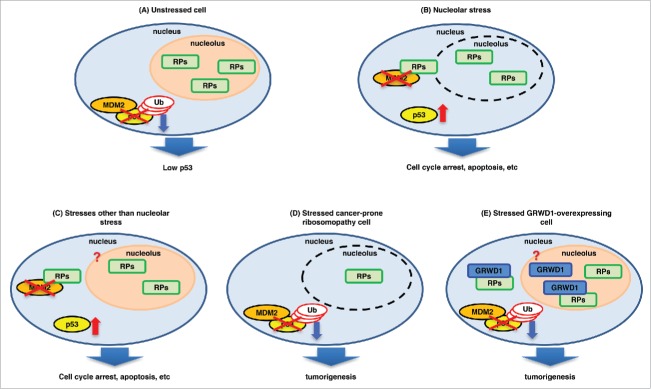
Figure 1.
A model for p53 regulation by ribosomal/nucleolar proteins. (A) In unstressed cells, tumor suppressive ribosomal proteins (RPs) such as RPL11 localize in the nucleolus, and MDM2 binds to and ubiquitinates p53. As a result, the cellular p53 level is kept low. (B) Nucleolar stress disrupts the nucleolus and releases the RPs into the nucleoplasm, where they interact with MDM2 and inhibit its ubiquitin ligase activity, inducing p53. (C) Other stresses such as DNA double-strand breaks and hypergrowth stimuli (e.g., expression of activated RAS) are also suggested to induce release of the RPs into the nucleoplasm, contributing to p53 induction. It is unclear whether nucleolar breakdown occurs under such conditions. (D) In cancer-prone ribosomopathy cells, the levels of the RPs are decreased by heterozygous mutations (or homozygous mutations for some factors). As a result, the RP-MDM2-p53 pathway cannot function in stressed cells, leading to tumorigenesis. (E) When overexpressed, GRWD1 binds to and inhibits RPL11, thereby down-regulating p53. As a result, tumor formation is promoted. As detailed in the text, PICT1 similarly binds to RPL11 and thereby inhibits p53.