Fig. 7.
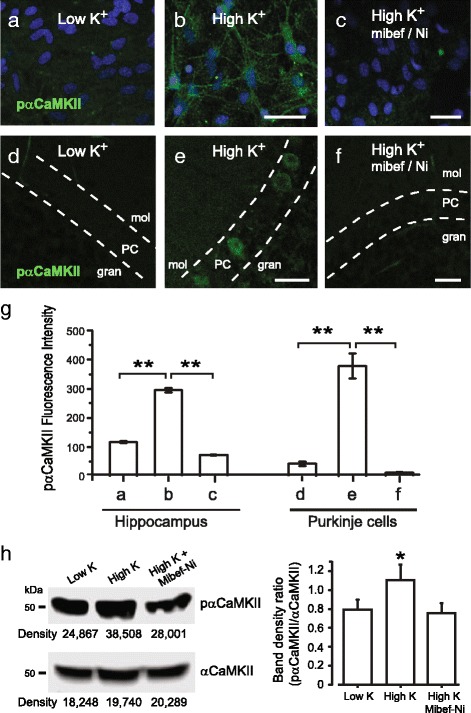
Cav3 calcium influx triggers αCaMKII phosphorylation in neurons. a-f Confocal images of cultured mouse hippocampal cells (a-c), and rat cerebellar tissue slices (d-f). Activated αCaMKII is identified by using a phospho-αCaMKII (pαCaMKII) antibody with additional DAPI label for nuclear staining in (a-c). Each preparation was maintained at rest in low [K]o (1 mM) or exposed to 10 min of high [K]o (50 mM) prior to fixation within 10 min of the end of high [K]o exposure. All results were derived from at least 3 separate experiments. In all neuronal cell tests calcium influx is restricted to Cav3 channels by maintaining cells in 30 μM Cd2+, 10 μM DNQX, 25 μM DL-AP5, and 1 μM TTX. a, b In hippocampal cell cultures high [K]o induces pαCaMKII labeling. d, e In cerebellar tissue slices high [K]o induces pαCaMKII labeling in Purkinje cell bodies. c, f High [K]o-induced labeling for pαCaMKII is blocked in both hippocampus (c) and cerebellum (f) in the presence of Cav3 channel blockers 1 μM mibefradil and 300 μM Ni2+. Dashed lines in (d-f) depict the boundaries of the Purkinje cell body layer. g Bar plots of the mean intensity of pαCaMKII fluorescence in hippocampal cell cultures and Purkinje cells in cerebellar tissue slices for panels (a-f) were quantified using ImageTrak software. Scale bars 10 μm. Values are mean ± SEM derived from n = 3–4 coverslips with 30–130 ROIs. h Western blot analysis to test the relative density of αCaMKII and p-αCaMKII labeling in cerebellar slice lysates in low or high [K]o, with mean bar plots of the band density ratio (pαCaMKII/αCaMKII) (Image J software). The high [K]o lysate has a higher band density than either low [K]o or high [K]o in the presence of mibefradil and Ni2+. Values are mean ± SEM derived from 3 separate experiments. * p < 0.05;** p < 0.01. PC, Purkinje cell layer; mol, molecular layer; gran, granule cell layer
