Abstract
Objective: Primary ovarian mucinous tumors progress from benign adenoma to borderline tumors to invasive mucinous carcinoma. A proper differential diagnosis is crucial to discriminate malignancies at the early stages of disease. However, few biomarkers are clinically available. We designed this study to analyze the clinical application of the oncogene IMP3 in monitoring early malignancies in ovarian primary mucinous tumors. Methods: We collected 250 samples of ovarian primary mucinous tumors along with the corresponding clinicopathological information between 2009 and 2015 at the Gynecology and Obstetrics Hospital of Fudan University and performed immunochemical assays. Statistical analysis of the correlation between expression of IMP3 and clinic-pathological parameters as well as the survivals of these patients was carried out. Finally, wound-healing and transwell assays were performed in SKOV3 and CAOV3 cells. Results: The expression rate and intensity of IMP3 were much higher in invasive carcinoma than those in benign adenoma and classic borderline adenoma (P<0.05). The expression rate and intensity of IMP3 were also higher in cases with mucinous intraepithelial carcinoma than those in cases with classic borderline tumors (P<0.05). Among the malignant cases, the expression rate and intensity of IMP3 increased with advancing FIGO staging (P<0.05). The expression rate and intensity of IMP3 were much higher in cases with involved fallopian tubes, uterine and omentum than those in cases without the involvement of these tissues (P<0.05). The expression rate and intensity of IMP3 were much higher in cases with lymph node metastasis than those in cases without lymph node metastasis (P<0.05). Elevated expression of IMP3 significantly deteriorated the disease-free survival (DFS) and overall survival (OS) of mucinous carcinoma (P<0.05). IMP3 was an independent risk factor of DFS but not OS. Further in vitro experiments indicated that IMP3 promoted the proliferation, motility and invasive potential of ovarian tumor cells. Conclusions: IMP3 is highly expressed in ovarian mucinous tumors and is positively correlated with malignancy. IMP3 could be used in the differential diagnosis of ovarian mucinous tumors and might be applicable in monitoring tumor initiation and progression.
Keywords: IMP3, ovarian mucinous tumor, immunochemistry, invasion
Introduction
Ovarian epithelial cancer is the leading malignancy in the female genital tract system with highly invasiveness and metastatic features. The 5-year survival for ovarian epithelial cancer is reported to be only 37% [1], suggesting that early screening and preventing the development of malignancies are crucial.
The primary mucinous tumor is one of the major histological subtypes of ovarian epithelial cancer [2-4] and accounts for approximately 3% of all ovarian epithelial cancers [2-4]. It is currently believed that the mucinous tumor belongs to the type I ovarian tumors, which progresses from benign to borderline to carcinoma [2]. Although most infiltrative mucinous carcinomas are stage I (based on the FIGO system), the recurrence rate is 20%, and overall survival is 14% once the tumor recurs and advances to higher stages [4]. It is of clinical significance to identify tumors with increased risk of malignancy. In contrast to other techniques for detecting tumors [5], immunochemistry is a more common tool in the differential diagnosis in daily practice. However, few biomarkers have been used to monitor early malignancies in primary mucinous tumors.
The insulin-like growth factor mRNA-binding protein 3 (IMP3) is a member of an evolutionarily conserved mRNA-binding protein family that regulates mRNA transport, translation and turnover [6]. Along with its family members IMP1 and IMP2, IMP3 is primarily expressed during the early stages of embryogenesis [7] and contributes to RNA trafficking and stabilization, cell growth, and cell migration. Growing evidence has demonstrated that IMP3 is an oncofetal protein that enhances the invasive abilities of tumor cells. IMP3 has been reported to be overexpressed in a variety of human malignancies, including ovarian, lung and gastrointestinal carcinomas [8-10], which makes this protein a reliable biomarker to distinguish some cancers from their benign mimetic lesions [11-13]. Previous studies have reported that IMP3 is abnormally expressed in other subtypes of ovarian carcinomas such as serous and clear cell. However, no studies have concentrated on IMP3 expression in primary ovarian mucinous tumors, raising the question of whether IMP3 can be used to identify malignancies in ovarian mucinous tumors. In this study, we used the immunohistochemistry to determine whether IMP3 is detectable and can be used to trace the early malignancy of mucinous tumors.
Methods
Patients and tissue handling
A total of 250 patients who underwent radical surgical resection for primary ovarian mucinous tumor at the Obstetrics and Gynecology Hospital of Fudan University between January 2009 and March 2015 were retrospectively evaluated. The study group included 100 cases of ovarian primary mucinous adenoma, 100 cases of borderline mucinous adenoma (classic and intraepithelial carcinoma) and 50 cases of malignant ovarian primary mucinous carcinoma. All the patients were diagnosed according to WHO classifications of the female genital system of 2014. All the cases were revised again by two pathologists who were blinded to the cancer status of the cases. We collected paraffin-embedded blocks containing the most obvious lesions to obtain consecutive tissue slides. This study was approved by the Clinical Research Ethics Committee of Obstetrics and Gynecology Hospital of Fudan University. Written informed consent was obtained from each participant involved in this study.
Clinical and demographic data including, patient age, tumor size, involved organs (including uterus, appendix and omentum), the lymph node status and the FIGO stage were extracted from the medical records, pathology report or discharge summary.
Immunohistochemistry and scoring
Tissue sections (3-4 μm thickness) were deparaffinized in xylene, rehydrated in a graded series of alcohol solutions and washed three times with PBS (pH=7.4, Gibco, Thermo Fisher, Massachusetts, USA). After antigen retrieval was performed (6 min in 100°C boiling water with 0.01 M citrate, pH 6.0), the slides were incubated in a solution of 0.3% hydrogen peroxide in phosphate-buffered saline for 15 min to block endogenous peroxidase activity. Next, the slides were incubated with a primary antibody targeting IMP3 (ab111072, Abcam, Hong Kong, China) at a 1:100 dilution overnight at 4°C and followed by EnVision secondary antibody system (Dako, Agilent, USA) at room temperature for 30 min. Visualization was achieved using diamino-benzidine. Validation of the IMP3 antibody has been previously described [9]. Adjacent normal bronchial epithelium within each tissue section served as an internal reference.
The staining results were evaluated by two independent experienced pathologists specialized in gynecology. IMP3 expression was scored using a combined system described in previous studies [9,13,14]: the staining intensity of IMP3 was categorized as none, weak, moderate or strong. Then, the scores were calculated by the following formula: 3 × percentage of strongly staining cells + 2 × percentage of moderately staining cells + percentage of weakly staining cells, which ranged from 0-300. Cases with scores between 0 and 49 were interpreted as negative (0), 50 and 99 as mildly positive (low), and 100 and 300 as moderate to strongly positive (high).
Cell lines and culture conditions
The human ovarian cell lines SKOV3 and CAOV3 were purchased from the Fudan University IBS Cell Bank (Shanghai, China). All cell lines were cultured in RPMI 1640 (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco), 50 U/mL penicillin and 50 μg/mL streptomycin (Gibco) at 37°C in a humidified atmosphere containing 5% CO2.
Plasmids
The full-length IMP3 sequence was amplified using PCR from cDNA obtained from HEK293T cells provided by the NIH and subcloned into the pcDNA3.1 (+) vector (Transheep, Shanghai, China).
Antibodies and reagents
The following antibodies were used: IMP3 (#57145) and GAPDH (D16H11, #5174) from Cell Signaling Technology (Boston, Massachusetts, USA). The following reagents were used: Lipofectamine 3000 transfection reagent (Lot. 11668-027, Invitrogen, Carlsbad, CA, USA) and RIPA lysis buffer (Lot. 89901, Thermo Scientific, USA).
Western blot
The western blot analysis was performed as previously described [14]. The cell lysates were boiled in loading buffer at 95°C for 5 min, separated on SDS-PAGE gels at 80 V for 2 h and then transferred to PVDF membranes for 2 h. Next, the membranes were blocked in 5% BSA for 30 min followed by incubation with primary antibodies at 4°C overnight. The membranes were then washed in 1% TBST three times and incubated with secondary antibodies for 1 h. Detection of the substrates was achieved using an ECL system (#32209, Thermo Fisher, USA).
RNA preparation, reverse transcription, and quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the gastric cancer cell lines using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Real-time (RT) and quantitative polymerase chain reaction (qPCR) kits were used to evaluate IMP3 expression in those cell lines. The RT-qPCR experiments were performed as previously described [15]. Relative gene expression levels were calculated using the comparative cycle threshold (CT) (2-ΔΔCT) method, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control to normalize the data. The following primers were used in this study: IMP3, forward AGACACCTGATGAGAATGACC and reverse GTTTCCTGAGCCTTTACTTCC; and GAPDH, forward GAGTCAACGGATTTGGTCGT and reverse GATCTCGCTCCTGGAAGATG.
Cell mobility and invasive assays
Cell mobility was assessed using wound-healing assays. SKOV3 and CAOV3 cells were transfected with either pcDNA3.1-IMP3 or pcDNA3.1 vectors using Lipofectamine 3000 transfection reagent (Invitrogen, Carlsbad, CA, USA). The cells were seeded into 6-well plates and grown to 100% confluence. Wounds were made by scratching the cell monolayer with a sterile pipette tip. The cells were then washed with PBS and maintained in serum-free culture medium. Wound closing progression was observed after the initial scratch and once every 24 h, and images were captured at 100 × magnification under a microscope (Olympus, Japan).
A transwell assay was used to assess the invasive potential of the cells. A total of 3 × 104 cells in serum-free medium was seeded into the upper insert of a Transwell system (Corning, USA), and 500 μL of culture medium containing 20% FBS was loaded into the lower chamber. After a 24-h incubation at 37°C, the cells that had migrated through the filters were fixed with ethanol, stained with crystal violet, photographed at 200 ×, and counted at 400 × under a microscope (Olympus).
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (IBM, SPSS, Chicago, IL, USA). Associations between the expression levels of IMP3 and the clinicopathological parameters were examined using the Spearman rank order analysis. DFS and OS curves were calculated using the Kaplan-Meier method and were analyzed with the log-rank test. DFS was calculated from the date of surgery to the date of disease progression (local and/or distal tumor recurrence) or to the date of death. The OS rate was defined as the length of time between the diagnosis and either death or last follow-up. A multivariate analysis was performed using the Cox proportional hazards model to evaluate the independent prognostic factors for survival in ovarian mucinous carcinoma. Variables with a value of P<0.05 in the univariate analysis were used in the multivariate analysis. All p values were 2-sided, and statistical significance was established at P<0.05.
Results
Patient characteristics
A total of 250 patients from 2009-2015 were recruited in our study. The ages of the patients ranged from 17 to 77 years, with an average age of 46.1±7.13. Among them, 100 (40%) had mucinous adenoma, 100 (40%) had borderline mucinous tumor (including classic and intraepithelial carcinoma), and 50 (20%) had invasive mucinous carcinoma (Figure 1A). In addition, the borderline mucinous tumors were subdivided into 70 (70%) classic borderline mucinous tumors and 30 (30%) intraepithelial carcinomas (Figure 1A). All the borderline tumors were of the intestinal type, and all the mucinous carcinomas were of the intestinal type. The average tumor size was 11.29±4.37 cm. We also collected the survival data from the mucinous carcinoma group (n=50). The median follow-up time for the surviving patients at the endpoint for analysis was 45 months. Five of the 50 patients died during follow-up.
Figure 1.

The morphology and immunochemical measurements of IMP3 expression in ovarian mucinous tumors. A: Representative Hematoxylin-Eosin staining of a series of ovarian mucinous tumors: benign mucinous cystoadenoma, classic borderline mucinous tumor, borderline mucinous tumor of intraepithelial carcinoma and invasive mucinous carcinoma. All the images were captured under a microscope at 200 × (Scale bar =50 μm, Top) and 400 × (Scale bar =20 μm, Bottom) magnification. B: The staining intensities of IMP3 expression based on our scoring system. a: None. b: Weak. c: Moderate. d: Strong. All the images were captured under a microscope at 400 × magnification (Scale bar =20 μm).
Different IMP3 immunostaining patterns in ovarian mucinous tumors
The staining of IMP3 in the ovarian mucinous tumors was localized to the cytoplasm, which was consistent with that in previous studies analyzing other tissues [15,16]. Then, we divided the intensity of the IMP immunostaining into none, weak, moderate and strong expression groups (Figure 1B). Based on this, we further scored all the cases according to the scoring system mentioned in the Methods section and in previous studies [9,13,14]. Patients with benign mucinous cystoadenoma (n=100) mainly presented negative IMP3 expression (88/100, 88%), while 12 cases exhibited weakly positive staining as indicated by the low final scores (scores 49-99, 12/100, 12%, Table 1). None of the cases presented either moderate or strong staining for IMP3. The cohort of classic borderline adenoma (n=100) cases presented more samples with weak IMP3 expression than the benign cystoadenoma cohort (55/70, 78.57%, P<0.05, Figure 2A), and 9 cases of classic borderline tumor exhibited moderate staining for IMP3 (scores 100-300, 9/70, 12.86%). The moderate positive areas in these cases were focally distributed, and the percentage of positive areas was below 20%. In contrast, in the mucinous intraepithelial carcinoma cohort (n=30), more cases showed moderately positive staining and the percentage of the area stained positive was between 50-75% (score 100-300, 14/30, 46.67%). The expression rate and intensity of IMP3 were much higher in cases of mucinous intraepithelial carcinoma than those in cases of classic borderline tumor (P<0.05, Table 1; Figure 2A, 2B). As expected, the highest rate of IMP3 expression was seen in mucinous carcinoma (n=50), as 64% of the cases (32/50) showed strong and diffuse staining for IMP3 (score 100-300) that was significantly higher than that in either classic borderline mucinous tumors or benign mucinous tumors (P<0.05, Table 1; Figure 2A, 2B). Interestingly, the expression of IMP3 failed to show a significant difference between intraepithelial carcinoma and invasive mucinous carcinoma. However, when compared with carcinomas of FIGO stage II-III, the expression of IMP3 was significantly higher in invasive carcinoma than that in intraepithelial carcinoma (P<0.05). Taken together, these results suggested that the expression rate and intensity of IMP3 increased as tumors progressed toward malignancy.
Table 1.
The correlation between IMP3 and the clinicopathological parameters of ovarian mucinous tumors
| Variables | Total | IMP3 | Rho | P value | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Negative | Low | High | |||||
| Age | <50 | 143 | 59 | 49 | 35 | 0.022 | 0.734 |
| ≥50 | 107 | 36 | 51 | 20 | |||
| Histology | Benign | 100 | 88 | 12 | 0 | 0.824 | <0.001* |
| Borderline | 70 | 6 | 55 | 9 | |||
| Intraepithelial carcinoma | 30 | 1 | 15 | 14 | |||
| Invasive carcinoma | 50 | 0 | 18 | 32 | |||
| Size | ≥10 cm | 167 | 73 | 67 | 27 | 0.213 | 0.001* |
| <10 cm | 83 | 22 | 33 | 28 | |||
p<0.01.
Figure 2.
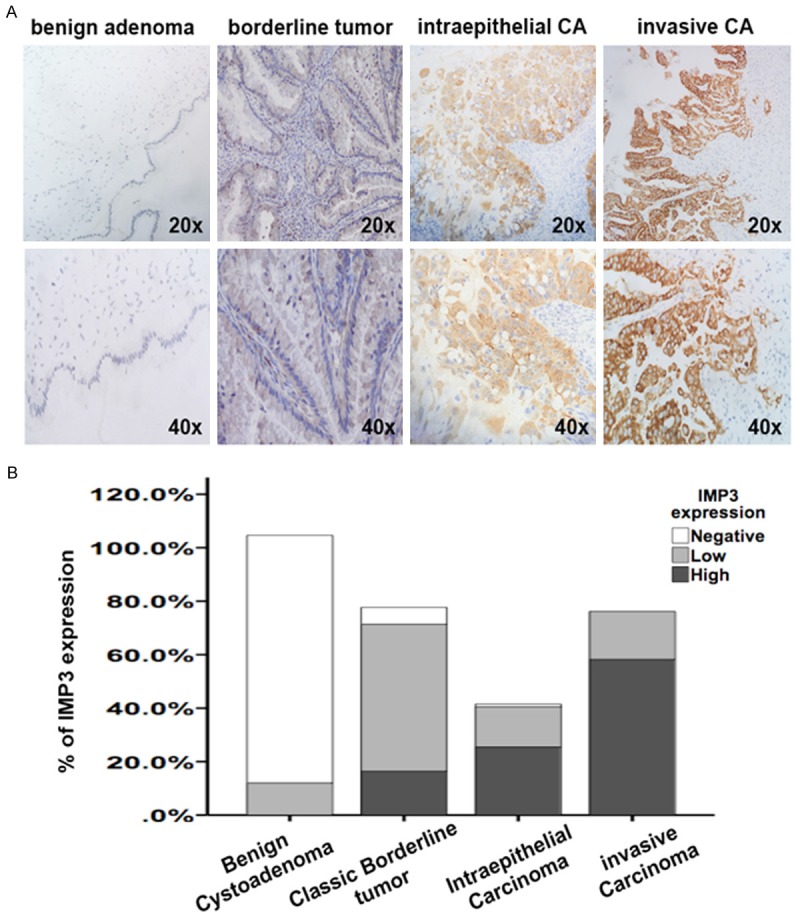
The expression of IMP3 in a series of ovarian mucinous tumors. A: Representative immunochemistry results of IMP3 in a series of ovarian mucinous tumors: benign mucinous cystoadenoma, classic borderline mucinous tumor, borderline mucinous tumor of intraepithelial carcinoma and invasive mucinous carcinoma. All the images were captured under a microscope at 200 × (Scale bar =50 mm, Top) and 400 × (Scale bar =20 mm, Bottom) magnification. B: The graph of percentage of different IMP3 expression levels in a series of ovarian mucinous tumors: benign mucinous cystoadenoma, classic borderline mucinous tumor, borderline mucinous tumor of intraepithelial carcinoma and invasive mucinous carcinoma. IMP3 expression was measured using immunochemistry and then classified as negative, low or high according to our scoring system.
Correlation between the expression level of IMP3 and clinicopathological characteristics
We analyzed the association between IMP3 expression and clinicopathological features. As shown in Table 1, IMP3 expression levels were significantly correlated with the tumor size (P<0.001, Table 1) in all cases, suggesting that IMP3 might be involved in the growth of ovarian mucinous tumors. Interestingly, when limiting the samples to malignant cases (mucinous carcinoma, n=50), the expression of IMP3 was not correlated to tumor size (Table 2). Next, in 50 cases with primary ovarian mucinous carcinoma, we found that IMP3 was significantly correlated with FIGO staging (P=0.005, Table 2). In detail, the expression rate and intensity of IMP3 were much higher in cases with fallopian (P=0.047) and uterine (P=0.020) involvement. Moreover, IMP3 expression was tightly correlated with lymph node metastasis (P=0.047) and recurrence (P=0.015) (Table 2). Taken together, the correlations between IMP3 expression and these clinicopathological indexes suggested that IMP3 might be associated with tumor invasion in ovarian mucinous malignancies.
Table 2.
The correlation between IMP3 and the clinicopathologic parameters of ovarian mucinous carcinoma
| Variables | IMP3 | X2 | P value | ||
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| FIGO staging | I | 16 | 17 | 10.627b | 0.005* |
| II | 2 | 6 | |||
| III and IV | 0 | 9 | |||
| Tumor size | ≥10 cm | 10 | 14 | 0.257a | 0.557 |
| <10 cm | 8 | 18 | |||
| Fallopian involvement | - | 16 | 21 | 3.950a | 0.047* |
| + | 1 | 12 | |||
| Uterine involvement | - | 17 | 22 | 5.452a | 0.020* |
| + | 0 | 11 | |||
| Omental involvement | - | 17 | 27 | 2.002a | 0.157 |
| + | 0 | 6 | |||
| Appendix involvement | - | 17 | 29 | 0.896a | 0.344 |
| + | 0 | 4 | |||
| Lymph node metastasis | - | 17 | 24 | 3.957a | 0.047* |
| + | 0 | 9 | |||
| Recurrence | - | 15 | 16 | 5.932a | 0.015* |
| + | 2 | 17 | |||
p<0.05.
Continuous correction.
Likelihood ratio, LR.
Correlation between the expression levels of IMP3 and survival in malignant mucinous carcinoma patients
To explore the potential correlation between IMP3 and the prognosis of patients with ovarian mucinous carcinoma, we conducted a Kaplan-Meier analysis in the subgroup of 50 malignant cases. The results showed that high levels of IMP3 expression were significantly associated with a worse DFS (P=0.004; Figure 3A) and OS (P=0.009; Figure 3B). Moreover, the Cox univariate analysis revealed that IMP3 levels were the risk factor for DFS (P<0.001) and OS (P<0.001) along with FIGO staging and lymph node metastasis (Tables 3, 4). The multivariate analysis showed that IMP3 levels were an independent risk factor for DFS but not for OS. Taken together, these results identified that high levels of IMP3 predicted poor survival of malignant mucinous carcinoma patients.
Figure 3.

IMP3 predicts a poor prognosis in invasive ovarian mucinous carcinoma. A: Kaplan-Meier disease-free survival (DFS) curves for cases of invasive ovarian mucinous carcinoma with different IMP3 levels (low, n=18 vs. high, n=32). B: Kaplan-Meier overall-survival (OS) curves for cases of invasive ovarian mucinous carcinoma with different IMP3 levels (low, n=18 vs. high, n=32).
Table 3.
Univariate and multivariate analyses of the clinicopathological factors for DFS
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p value | HR | 95% CI | P value | |
| Age | 1.459 | 0.576-3.698 | 0.425 | |||
| Tumor size | 1.084 | 0.439-2.676 | 0.861 | |||
| Lymph node metastasis | 14.844 | 4.874-45.208 | <0.001* | -- | -- | -- |
| FIGO staging | 6.359 | 3.066-13.190 | <0.001* | 3.375 | 1.163-9.799 | 0.025* |
| IMP3 | 6.706 | 2.779-16.182 | <0.001* | 2.909 | 1.143-7.406 | 0.025* |
p<0.05.
HR: hazard ratio.
Table 4.
Univariate and multivariate analyses of the clinicopathological factors for OS
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p value | HR | 95% CI | P value | |
| Age | 1.486 | 0.547-4.039 | 0.438 | |||
| Tumor size | 2.408 | 0.845-6.866 | 0.100 | |||
| Lymph node metastasis | 16.077 | 4.829-53.531 | <0.001* | -- | -- | -- |
| FIGO staging | 6.902 | 3.092-15.407 | <0.001* | 4.234 | 1.431-12.527 | 0.009* |
| IMP3 | 4.809 | 2.158-10.719 | <0.001* | -- | -- | -- |
p<0.05.
HR: hazard ratio.
Overexpression of IMP3 promotes the proliferation, mobility and invasion of ovarian tumor cells
Since high expression of IMP3 was correlated with tumor size and invasiveness in earlier analyses, we performed in vitro experiments to identify its effect on cell function in ovarian tumor cells. We first detected the baseline IMP3 mRNA and protein levels in 5 ovarian tumor cell lines and selected SKOV3 and CAOV3 for the experiments (Figure 4A, 4B). Then, we constructed the IMP3 plasmid, transfected it into SKOV3 and CAOV3 cells and tested the transfection efficiency at the mRNA and protein levels (Figure 4C, 4D). We found that overexpression of IMP3 caused significantly accumulation of live SKOV3 and CAOV3 cells (Figure 4E, 4F, P<0.01). In addition, overexpression of IMP3 prompted the invasion of SKOV3 and CAOV3 cells in the transwell assays (Figure 5A, 5B, P<0.05). Similarly, overexpression of IMP3 also enhanced cell migration in the wound-healing assays in both cell lines (Figure 5C, 5D, P<0.05). These results suggested that IMP3 promotes cell proliferation, migration and invasion in ovarian tumor cells.
Figure 4.
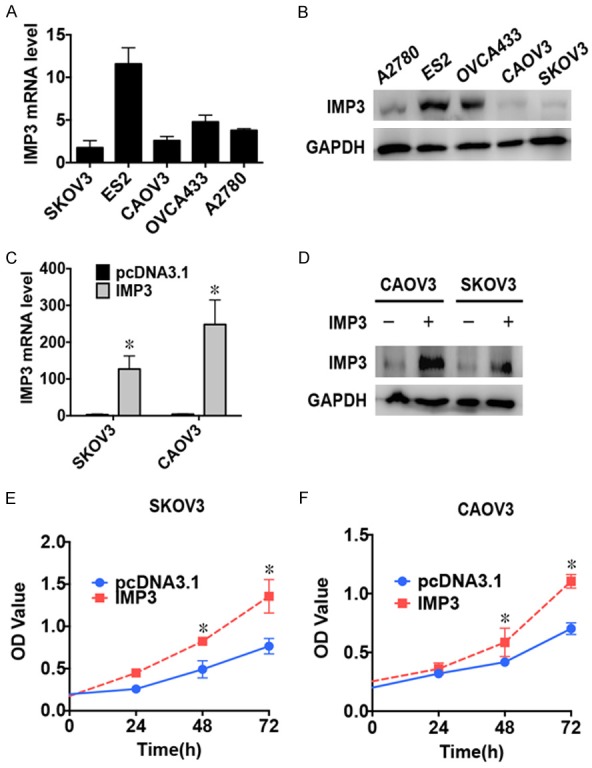
Overexpression of IMP3 induces tumor cell proliferation in ovarian tumor cell lines. A: Graph showing the statistical analysis of the baseline IMP3 mRNA levels in the 5 ovarian tumor cell lines as detected by RT-qPCR. B: Results of the western blot analysis showing baseline IMP3 levels in 5 ovarian tumor cell lines. GAPDH was used as an endogenous control. C: Graph showing the statistical analysis of IMP3 mRNA levels in SKOV3 and CAOV3 cells transfected with pcDNA3.1-IMP3 as detected using RT-qPCR. *: P<0.01. D: Results of the western blot analysis showing IMP3 levels in SKOV3 and CAOV3 cell lines transfected with pcDNA3.1-IMP3. GAPDH was used as an endogenous control. E: The CCK8 cell counting assays indicate the cell growth curves of SKOV3 cells transfected with either pcDNA3.1 or pcDNA3.1-IMP3 at the indicated time points. Error bars are shown. *: P<0.01. F: The CCK8 cell counting assays indicate the cell growth curves of CAOV3 cells transfected with either pcDNA3.1 or pcDNA3.1-IMP3 at the indicated time points. Error bars are shown. *: P<0.01.
Figure 5.
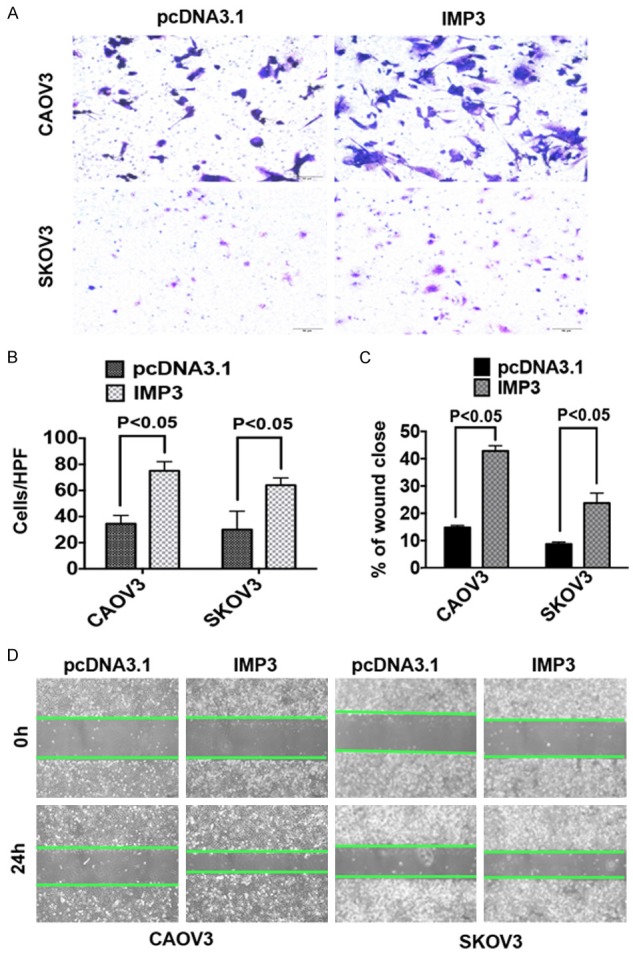
IMP3 promotes tumor cell migration and invasion in ovarian tumor cells. (A, B) Representative images (A) and quantification (B) of the transwell assays performed using SKOV3 and CAOV3 cells transfected with either pcDNA3.1 or pcDNA3.1-IMP3. Images were captured under a microscope at 200 × magnification (scale bars =50 μm, P<0.05). (C, D) Representative quantification (C) and images (D) of wound-healing assays using SKOV3 and CAOV3 cells transfected with either pcDNA3.1 or pcDNA3.1-IMP3. Images were captured under a microscope at 40 × magnification (scale bars =200 μm, P<0.05).
Discussion
To the best of our knowledge, this is the first study to investigate the expression of IMP3 in ovarian mucinous tumors and its relationship with clinicopathological variables and patient prognosis. We found that the expression rate and intensity of IMP3 gradually increased along with the progression of ovarian mucinous tumors in a stepwise manner. Elevated expression of IMP3 was significantly associated with a worse DFS and OS in cases of mucinous carcinoma. Moreover, IMP3 overexpression promoted cell proliferation and invasion in ovarian tumor cells. Thus, we identified IMP3 as a diagnostic and prognostic biomarker for ovarian mucinous tumors.
Ovarian borderline mucinous tumors were originally divided into two histological subtypes: the gastrointestinal type and the seromucinous type. The seromucinous type was independently categorized according to the WHO 2014 criteria; thus, only borderline mucinous tumors of the intestinal type were included in our study. Borderline mucinous tumors from the ovary are microscopically identified as cysts lined by stratified, proliferative gastrointestinal-type mucinous epithelium exhibiting papillary intraglandular growth patterns without stromal invasion [17]. The difference between classic borderline mucinous tumors and intraepithelial carcinoma is the severe nuclear atypia. Meanwhile, only intraepithelial carcinoma with stromal invasion measuring more than 5 mm at the longest diameter would be considered mucinous carcinoma [17-19]. The group with stromal invasive lesions smaller than 5 mm at the longest diameter is separately identified as intraepithelial carcinoma with microinvasion because its behavior has not been well documented to date [17]. Thus, this group was not included in our study because of its ambiguous identity between typical intraepithelial carcinoma and well-defined mucinous carcinoma.
IMP3 is not expressed in most mature normal tissues but is abnormally regulated in a variety of cancers, which supports its identity as an oncogene [2,8,13]. IMP3 was reported to be significantly associated with invasive progression of various tumor types [20-22]. In the gynecological system, IMP3 has been used as a novel biomarker to distinguish cancer (especially borderline cases) from normal tissues within the cervix and endometrium [23,24]. Yiying Wang’s research group reported that IMP3 was involved in the initial process of early serous tubal carcinogenesis and was more often abnormally increased in patients with high-grade serous carcinoma [13]. Similarly, we also found that the expression of IMP3 increased significantly as the cancer progressed from a benign to malignant state and was significantly correlated with FIGO staging. The distinctive expression of IMP3 in classic borderline and intraepithelial carcinoma suggested that IMP3 accumulates with the increasing malignancy of ovarian mucinous tumors. Interestingly, we found no significant differences in IMP3 expression between intraepithelial carcinoma and invasive mucinous carcinoma. Since IMP3 expression was significantly correlated with FIGO staging and invasion-related indexes and we observed that IMP3 overexpression promoted cell invasion in the in vitro experiments, future studies should focus on the innate differences in IMP3 expression between invasive mucinous carcinoma and intraepithelial carcinoma with a larger cohort.
It was previously suggested that IMP3 expression might be histological subtype-specific because high expression levels were observed in serous and clear cell carcinoma compared with those in endometrioid carcinomas [2,11]. Our study found that IMP3 was also expressed in primary mucinous carcinoma and was correlated with FIGO staging. Further statistical analyses also suggested that IMP3 expression was highly correlated with tumor size, tumor invasion-related indexes and lymph node metastasis. Since a big percentage of endometrioid and mucinous carcinomas are of FIGO stage I, it is more likely that IMP3 expression is correlated with the progression toward malignancy rather than the specificity of histological subtypes.
In the present study, patients with high levels of IMP3 expression had a poor prognosis, which is consistent with findings on ovarian clear cell carcinoma [11]. We demonstrated that IMP3 could serve as an independent risk marker for DFS but not for OS in mucinous carcinoma. Considering that the sample size was limited and there were fewer cases of advanced stage disease than those of early stage disease, the statistical results might not reflect the innate connection of IMP3 and the prognosis of mucinous carcinoma. Future studies should be established with a larger cohort and identical numbers of early and advanced stage cases to more precisely elucidate the link between IMP3 and the clinical consequences of invasive ovarian mucinous carcinoma.
It has been reported that IMP3 could stimulate tumor proliferation and invasion in somatic malignancies [21,26]. Our clinical data and in vitro experiment results also suggested that IMP3 was correlated with tumor size in all types of ovarian mucinous tumors and promoted cell proliferation in ovarian tumor cells. On the other hand, high levels of IMP3 expression were correlated with tumor invasion in the clinical data analysis, and we identified that overexpression of IMP3 could stimulate tumor cell migration and invasion in vitro. A previous study reported that EGF could regulate IMP3 to enhance tumor invasion [20]. Future studies should focus on the underlying molecular mechanisms.
In summary, we found that IMP3 is detectable throughout the entire spectrum of ovarian mucinous tumors. IMP3 is highly expressed with increasing malignancy of ovarian mucinous tumors. IMP3 expression is also an indicator of poor prognostic significance. Clinical practices should consider IMP3 as a biomarker in diagnosing ovarian mucinous tumors and monitoring patient prognosis.
Conclusions
IMP3 is highly expressed with increasing malignancy in ovarian mucinous tumors. IMP3 could be used in the differential diagnosis of ovarian mucinous tumors and might be applicable in monitoring tumor initiation and progression.
Acknowledgements
This Project was supported by the National Natural Science Foundation of China (Grant No. 81602269). We are grateful to the faculty at the Department of Pathology at the Obstetrics and Gynecology Hospital of Fudan University for preparing the paraffin slides for our study.
Disclosure of conflict of interest
None.
References
- 1.Anuradha S, Webb PM, Blomfield P, Brand AH, Friedlander M, Leung Y, Obermair A, Oehler MK, Quinn M, Steer C, Jordan SJ. Survival of Australian women with invasive epithelial ovarian cancer: a population-based study. Med J Aust. 2014;201:283–288. doi: 10.5694/mja14.00132. [DOI] [PubMed] [Google Scholar]
- 2.Chiste M, Alexis J, Recine M. IMP3 expression in serous tumors of the ovary. Appl Immunohistochem Mol Morphol. 2014;22:658–662. doi: 10.1097/PAI.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 3.Mhawech-Fauceglia P, Yan L, Liu S, Pejovic T. ER+/PR+/TFF3+/IMP3-immunoprofile distinguishes endometrioid from serous and clear cell carcinomas of the endometrium: a study of 401 cases. Histopathology. 2013;62:976–985. doi: 10.1111/his.12096. [DOI] [PubMed] [Google Scholar]
- 4.Danialan R, Assaad M, Burghardt J, Newcomb P, Cartun RW, Mandavilli S. The utility of PAX8 and IMP3 immunohistochemical stains in the differential diagnosis of benign, premalignant, and malignant endocervical glandular lesions. Gynecol Oncol. 2013;130:383–388. doi: 10.1016/j.ygyno.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79:1581–1586. doi: 10.1002/(sici)1097-0142(19970415)79:8<1581::aid-cncr21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–1270. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller-Pillasch F, Pohl B, Wilda M, Lacher U, Beil M, Wallrapp C, Hameister H, Knochel W, Adler G, Gress TM. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev. 1999;88:95–99. doi: 10.1016/s0925-4773(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 8.Damasceno EA, Carneiro FP, Magalhaes AV, Carneiro Mde V, Takano GH, Vianna LM, Seidler HB, Castro TM, Muniz-Junqueira MI, Amorim RF, Ferreira VM, Motoyama AB. IMP3 expression in gastric cancer: association with clinicopathological features and HER2 status. J Cancer Res Clin Oncol. 2014;140:2163–2168. doi: 10.1007/s00432-014-1850-9. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Wei P, Shen C, Yang Y, Wang Y, Li Y, Du X. Prognostic value of the IASLC/ATS/ERS classification and IMP3 expression in lung adenocarcinoma of Chinese cases. Am J Cancer Res. 2015;5:2266–2276. [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu KF, Shen MR, Huang YF, Cheng YM, Lin SH, Chow NH, Cheng SW, Chou CY, Ho CL. Overexpression of the RNA-binding proteins Lin28B and IGF2BP3 (IMP3) is associated with chemoresistance and poor disease outcome in ovarian cancer. Br J Cancer. 2015;113:414–424. doi: 10.1038/bjc.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobel M, Xu H, Bourne PA, Spaulding BO, Shih Ie M, Mao TL, Soslow RA, Ewanowich CA, Kalloger SE, Mehl E, Lee CH, Huntsman D, Gilks CB. IGF2BP3 (IMP3) expression is a marker of unfavorable prognosis in ovarian carcinoma of clear cell subtype. Mod Pathol. 2009;22:469–475. doi: 10.1038/modpathol.2008.206. [DOI] [PubMed] [Google Scholar]
- 12.Vercellini P, Cribiu FM, Del Gobbo A, Carcangiu ML, Somigliana E, Bosari S. The oncofetal protein IMP3: a novel biomarker and triage tool for premalignant atypical endometriotic lesions. Fertil Steril. 2013;99:1974–1979. doi: 10.1016/j.fertnstert.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Wen N, Wang Y, Wen L, Zhao SH, Ai ZH, Wang Y, Wu B, Lu HX, Yang H, Liu WC, Li Y. Overexpression of FOXM1 predicts poor prognosis and promotes cancer cell proliferation, migration and invasion in epithelial ovarian cancer. J Transl Med. 2014;12:134. doi: 10.1186/1479-5876-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Specht E, Kaemmerer D, Sanger J, Wirtz RM, Schulz S, Lupp A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology. 2015;67:368–377. doi: 10.1111/his.12662. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Li L, Wang Y, Yuan Z, Zhang W, Hatch KD, Zheng W. IMP3 as a cytoplasmic biomarker for early serous tubal carcinogenesis. J Exp Clin Cancer Res. 2014;33:60. doi: 10.1186/s13046-014-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Gobbo A, Vaira V, Guerini Rocco E, Palleschi A, Bulfamante G, Ricca D, Fiori S, Bosari S, Ferrero S. The oncofetal protein IMP3: a useful marker to predict poor clinical outcome in neuroendocrine tumors of the lung. J Thorac Oncol. 2014;9:1656–1661. doi: 10.1097/JTO.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg SG, Bell DA, Kurman RJ, Seidman JD, Prat J, Ronnett BM, Copeland L, Silva E, Gorstein F, Young RH. Borderline ovarian tumors: key points and workshop summary. Hum Pathol. 2004;35:910–917. doi: 10.1016/j.humpath.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002;26:139–152. doi: 10.1097/00000478-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Nomura K, Aizawa S, Hano H. Ovarian mucinous borderline tumors of intestinal type without intraepithelial carcinoma: are they still tumors of low malignant potential? Pathol Int. 2004;54:420–424. doi: 10.1111/j.1440-1827.2004.01645.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Jung IH, Hwang YS. EGF enhances low-invasive cancer cell invasion by promoting IMP-3 expression. Tumour Biol. 2016;37:2555–2563. doi: 10.1007/s13277-015-4099-2. [DOI] [PubMed] [Google Scholar]
- 21.Pasiliao CC, Chang CW, Sutherland BW, Valdez SM, Schaeffer D, Yapp DT, Ng SS. The involvement of insulin-like growth factor 2 binding protein 3 (IMP3) in pancreatic cancer cell migration, invasion, and adhesion. BMC Cancer. 2015;15:266. doi: 10.1186/s12885-015-1251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheen YS, Liao YH, Lin MH, Chu CY, Ho BY, Hsieh MC, Chen PC, Cha ST, Jeng YM, Chang CC, Chiu HC, Jee SH, Kuo ML, Chu CY. IMP3 promotes migration and invasion of melanoma cells by modulating the expression of HMGA2 and predicts poor prognosis in melanoma. J Invest Dermatol. 2015;135:1065–1073. doi: 10.1038/jid.2014.480. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Zota V, Woda BA, Rock KL, Fraire AE, Jiang Z, Lu D, Xu B, Dresser K, Lutman CV, Fischer AH. Expression of a novel oncofetal mRNA-binding protein IMP3 in endometrial carcinomas: diagnostic significance and clinicopathologic correlations. Mod Pathol. 2007;20:1263–1268. doi: 10.1038/modpathol.3800960. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Rock KL, Woda BA, Jiang Z, Fraire AE, Dresser K. IMP3 is a novel biomarker for adenocarcinoma in situ of the uterine cervix: an immunohistochemical study in comparison with p16 (INK4a) expression. Mod Pathol. 2007;20:242–247. doi: 10.1038/modpathol.3800735. [DOI] [PubMed] [Google Scholar]
- 25.Tabrizi AD, Kalloger SE, Kobel M, Cipollone J, Roskelley CD, Mehl E, Gilks CB. Primary ovarian mucinous carcinoma of intestinal type: significance of pattern of invasion and immunohistochemical expression profile in a series of 31 cases. Int J Gynecol Pathol. 2010;29:99–107. doi: 10.1097/PGP.0b013e3181bbbcc1. [DOI] [PubMed] [Google Scholar]
- 26.Hwang YS, Ahn SY, Moon S, Zheng Z, Cha IH, Kim J, Zhang X. Insulin-like growth factor-II mRNA binding protein-3 and podoplanin expression are associated with bone invasion and prognosis in oral squamous cell carcinoma. Arch Oral Biol. 2016;69:25–32. doi: 10.1016/j.archoralbio.2016.05.008. [DOI] [PubMed] [Google Scholar]


